Calcium phosphate is a highly essential compound in various fields, including biology, chemistry, and medicine. Its unique properties make it a crucial component in many applications, from food production to pharmaceuticals. One of the key characteristics of calcium phosphate is its molar mass, which is a critical parameter in understanding its behavior and interactions. In this article, we will delve into the world of calcium phosphate and explore its molar mass in just one minute.
What is Calcium Phosphate?
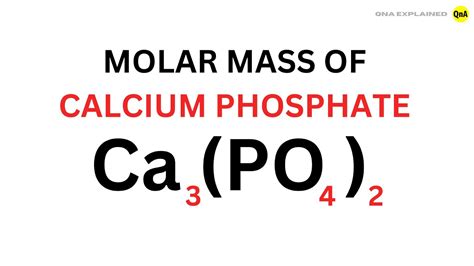
Before we dive into the molar mass of calcium phosphate, let's first understand what it is. Calcium phosphate is a chemical compound with the molecular formula Ca3(PO4)2. It is composed of calcium ions (Ca2+) and phosphate ions (PO43-), which are essential for many biological processes. Calcium phosphate is a major component of bones and teeth in humans and animals, and it plays a vital role in maintaining their structure and strength.
Importance of Molar Mass
The molar mass of a compound is a measure of its molecular weight, which is the sum of the atomic masses of its constituent atoms. In the case of calcium phosphate, its molar mass is crucial in understanding its physical and chemical properties, such as its solubility, melting point, and reactivity. The molar mass of calcium phosphate is also essential in calculating its concentration in solutions, which is critical in various applications, including food production, pharmaceuticals, and biomedical research.
Calculating the Molar Mass of Calcium Phosphate
The molar mass of calcium phosphate can be calculated by summing the atomic masses of its constituent atoms. The molecular formula of calcium phosphate is Ca3(PO4)2, which means it contains three calcium atoms, two phosphorus atoms, and eight oxygen atoms. The atomic masses of these elements are:
- Calcium (Ca): 40.08 g/mol
- Phosphorus (P): 30.97 g/mol
- Oxygen (O): 16.00 g/mol
Using these atomic masses, we can calculate the molar mass of calcium phosphate as follows:
Molar mass of Ca3(PO4)2 = (3 x 40.08) + (2 x 30.97) + (8 x 16.00) = 120.24 + 61.94 + 128.00 = 310.18 g/mol
Practical Applications of Calcium Phosphate's Molar Mass
The molar mass of calcium phosphate has several practical applications in various fields. For example:
- Food Production: Calcium phosphate is used as a food additive to enhance the texture and stability of various food products, including cheese, ice cream, and bread. Its molar mass is essential in calculating its concentration in these products.
- Pharmaceuticals: Calcium phosphate is used as an excipient in many pharmaceutical applications, including tablets, capsules, and injections. Its molar mass is critical in calculating its concentration in these formulations.
- Biomedical Research: Calcium phosphate is used as a biomaterial in various biomedical applications, including bone grafting and tissue engineering. Its molar mass is essential in understanding its interactions with biological systems.
Gallery of Calcium Phosphate





FAQs
What is the molecular formula of calcium phosphate?
+The molecular formula of calcium phosphate is Ca3(PO4)2.
What is the molar mass of calcium phosphate?
+The molar mass of calcium phosphate is 310.18 g/mol.
What are the practical applications of calcium phosphate's molar mass?
+The molar mass of calcium phosphate has practical applications in food production, pharmaceuticals, and biomedical research.
In conclusion, the molar mass of calcium phosphate is a critical parameter in understanding its physical and chemical properties, as well as its interactions with biological systems. Its practical applications in food production, pharmaceuticals, and biomedical research make it an essential compound in various fields. By understanding the molar mass of calcium phosphate, we can unlock its full potential and harness its unique properties to improve our daily lives.