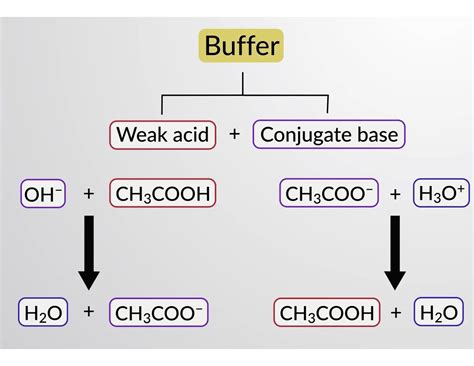
Buffer solutions are essential in various scientific and industrial applications, including laboratory settings, pharmaceutical manufacturing, and biological research. A buffer is a solution that resists changes in pH when acids or bases are added to it. One common buffer component is sodium hydroxide (NaOH). However, whether NaOH is a good buffer for your needs depends on several factors.
The importance of buffers in various applications cannot be overstated. In laboratory settings, buffers are crucial for maintaining the stability of chemical reactions and ensuring accurate results. In biological research, buffers help maintain the optimal pH for enzyme activity, protein stability, and cell growth. In pharmaceutical manufacturing, buffers are used to control the pH of formulations, which is critical for the stability and efficacy of the final product.

NaOH, also known as lye or caustic soda, is a strong base that is commonly used in various industrial applications, including the manufacture of paper, textiles, and detergents. In the context of buffers, NaOH is often used as a component of a buffer solution, typically in combination with a weak acid or its conjugate base. The choice of NaOH as a buffer component depends on the specific requirements of the application.
Benefits of Using NaOH as a Buffer Component
There are several benefits to using NaOH as a buffer component. One of the primary advantages is its high solubility in water, which makes it easy to prepare buffer solutions with precise pH values. Additionally, NaOH is a strong base, which means it can effectively neutralize acids and maintain a stable pH.

Another benefit of using NaOH is its relatively low cost compared to other buffer components. This makes it an attractive option for large-scale industrial applications where cost is a significant factor. Furthermore, NaOH is widely available and can be easily sourced from various suppliers.
Limitations of Using NaOH as a Buffer Component
While NaOH has several benefits as a buffer component, there are also some limitations to consider. One of the primary limitations is its high alkalinity, which can be problematic in certain applications. For example, in biological research, high alkalinity can be detrimental to cell growth and enzyme activity.

Another limitation of using NaOH is its potential to react with certain materials, such as glass or plastics. This can lead to contamination or degradation of the buffer solution, which can affect its performance and stability.
Alternatives to NaOH as a Buffer Component
Depending on the specific requirements of the application, there are several alternatives to NaOH as a buffer component. One common alternative is potassium hydroxide (KOH), which has similar properties to NaOH but is less alkaline. Another alternative is ammonium hydroxide (NH4OH), which is a weaker base than NaOH but can still provide effective buffering capacity.

Ultimately, the choice of buffer component depends on the specific requirements of the application. It is essential to consider factors such as pH range, buffer capacity, and compatibility with other materials when selecting a buffer component.
Conclusion
In conclusion, NaOH can be a good buffer component for certain applications, but it is essential to consider its limitations and potential drawbacks. By understanding the benefits and limitations of NaOH, researchers and manufacturers can make informed decisions about the best buffer component for their specific needs.






What is the primary function of a buffer component?
+The primary function of a buffer component is to resist changes in pH when acids or bases are added to a solution.
What are the benefits of using NaOH as a buffer component?
+The benefits of using NaOH as a buffer component include its high solubility in water, high alkalinity, and relatively low cost.
What are the limitations of using NaOH as a buffer component?
+The limitations of using NaOH as a buffer component include its high alkalinity, potential to react with certain materials, and compatibility issues with other components.