Water vapor pressure is an essential concept in various fields, including chemistry, physics, and engineering. It plays a crucial role in understanding the behavior of water in different conditions. In this article, we will delve into the world of water vapor pressure and explore five key facts about it, specifically at 20°C.
Water vapor pressure is the pressure exerted by water vapor in a system. It is an important parameter in determining the equilibrium between liquid and vapor phases. At 20°C, water vapor pressure has several unique characteristics that are worth exploring.
What is Water Vapor Pressure?
Before we dive into the key facts, let's understand what water vapor pressure is. Water vapor pressure is the pressure exerted by water molecules in the vapor phase. It is a measure of the force exerted by water molecules on the surface of a liquid or solid. The pressure is a result of the kinetic energy of the water molecules, which are constantly moving and colliding with each other.
At 20°C, the water vapor pressure is relatively low compared to higher temperatures. However, it is still an essential parameter in determining the behavior of water in various systems.

Key Fact #1: Water Vapor Pressure at 20°C is 2.34 kPa
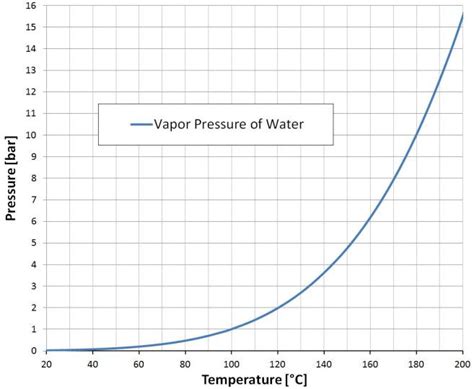
The first key fact about water vapor pressure at 20°C is its value. At this temperature, the water vapor pressure is approximately 2.34 kPa (kiloPascals). This value is relatively low compared to higher temperatures, where the water vapor pressure increases exponentially.
To put this value into perspective, the atmospheric pressure at sea level is approximately 101.3 kPa. This means that the water vapor pressure at 20°C is roughly 2.3% of the atmospheric pressure.

Key Fact #2: Water Vapor Pressure Increases with Temperature
The second key fact about water vapor pressure is its relationship with temperature. As the temperature increases, the water vapor pressure also increases. This is because higher temperatures provide more energy for the water molecules to move and collide with each other.
At 20°C, the water vapor pressure is relatively low, but as the temperature increases, the pressure increases exponentially. For example, at 30°C, the water vapor pressure is approximately 4.25 kPa, which is roughly 1.8 times the value at 20°C.

Key Fact #3: Water Vapor Pressure Affects Humidity
The third key fact about water vapor pressure is its impact on humidity. Humidity is the amount of water vapor in the air, and it is directly related to the water vapor pressure.
At 20°C, the water vapor pressure is relatively low, which means that the air can hold less water vapor. As the temperature increases, the water vapor pressure increases, allowing the air to hold more water vapor. This is why high temperatures often feel more humid than low temperatures.

Key Fact #4: Water Vapor Pressure Affects Evaporation Rates
The fourth key fact about water vapor pressure is its impact on evaporation rates. Evaporation is the process by which liquid water turns into vapor, and it is directly related to the water vapor pressure.
At 20°C, the water vapor pressure is relatively low, which means that the evaporation rate is slower. As the temperature increases, the water vapor pressure increases, allowing for faster evaporation rates.

Key Fact #5: Water Vapor Pressure is Essential for Climate Modeling
The fifth and final key fact about water vapor pressure is its importance in climate modeling. Water vapor pressure plays a critical role in determining the Earth's climate, and it is an essential parameter in climate models.
At 20°C, the water vapor pressure is relatively low, but it is still an important factor in determining the Earth's climate. Climate models use water vapor pressure data to predict future climate scenarios, and it is an essential parameter in understanding the Earth's climate system.

Gallery of Water Vapor Pressure






What is water vapor pressure?
+Water vapor pressure is the pressure exerted by water molecules in the vapor phase.
What is the value of water vapor pressure at 20°C?
+The value of water vapor pressure at 20°C is approximately 2.34 kPa.
How does water vapor pressure affect humidity?
+Water vapor pressure affects humidity by determining the amount of water vapor in the air.
In conclusion, water vapor pressure is an essential concept in understanding the behavior of water in different conditions. At 20°C, the water vapor pressure is relatively low, but it is still an important parameter in determining the Earth's climate. We hope that this article has provided you with a comprehensive understanding of water vapor pressure and its importance in various fields.