The NaCl model, also known as the sodium chloride model, is a fundamental concept in chemistry that helps us understand the structure and properties of ionic compounds. This model is closely related to Bohr's atomic theory, which revolutionized our understanding of the atomic structure. In this article, we will delve into the NaCl model and its connections to Bohr's atomic theory, exploring the insights it provides into the world of chemistry.
What is the NaCl Model?
The NaCl model, also known as the rock salt model, is a simple ionic model that describes the structure of sodium chloride (NaCl), commonly known as table salt. In this model, sodium ions (Na+) and chloride ions (Cl-) are arranged in a three-dimensional lattice structure, with each sodium ion surrounded by six chloride ions and each chloride ion surrounded by six sodium ions. This arrangement is known as a face-centered cubic (FCC) lattice.
Bohr's Atomic Theory
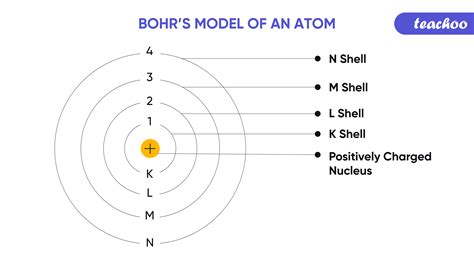
Niels Bohr's atomic theory, introduced in 1913, was a groundbreaking concept that challenged the existing understanding of the atomic structure. Bohr proposed that atoms consist of a small, dense nucleus surrounded by electrons that occupy specific energy levels or shells. The electrons in each shell have a specific energy, and when an electron moves from one shell to another, it absorbs or emits energy in the form of photons.
Relationship between the NaCl Model and Bohr's Atomic Theory
The NaCl model and Bohr's atomic theory are closely related, as the NaCl model is based on the principles of ionic bonding, which is a result of the electrostatic attraction between oppositely charged ions. In the NaCl model, the sodium ions (Na+) and chloride ions (Cl-) are formed when electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
According to Bohr's atomic theory, when a sodium atom loses an electron, it becomes a positively charged ion (Na+), while a chlorine atom gains an electron to become a negatively charged ion (Cl-). The electrostatic attraction between these oppositely charged ions leads to the formation of a strong ionic bond, which holds the sodium and chloride ions together in the NaCl lattice.

Insights into the NaCl Model
The NaCl model provides valuable insights into the structure and properties of ionic compounds. Some of the key insights include:
- Ionic bonding: The NaCl model demonstrates the concept of ionic bonding, where the electrostatic attraction between oppositely charged ions leads to the formation of a strong chemical bond.
- Lattice structure: The NaCl model shows how ions are arranged in a three-dimensional lattice structure, which is a fundamental concept in solid-state chemistry.
- Electrostatic attraction: The model highlights the importance of electrostatic attraction in holding ions together in a crystal lattice.
Bohr's Atomic Theory and the NaCl Model: A Deeper Connection
Bohr's atomic theory and the NaCl model are connected at a deeper level, as both concepts rely on the principles of quantum mechanics. The NaCl model can be seen as a manifestation of the quantum mechanical principles that govern the behavior of electrons in atoms and molecules.
In the NaCl model, the electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. This process can be understood in terms of the quantum mechanical concept of wave-particle duality, where electrons exhibit both wave-like and particle-like behavior.

Conclusion: Exploring the NaCl Model and Bohr's Atomic Theory
In conclusion, the NaCl model and Bohr's atomic theory are two fundamental concepts in chemistry that are closely related. The NaCl model provides valuable insights into the structure and properties of ionic compounds, while Bohr's atomic theory revolutionized our understanding of the atomic structure.
By exploring the connections between these two concepts, we gain a deeper understanding of the quantum mechanical principles that govern the behavior of electrons in atoms and molecules. The NaCl model and Bohr's atomic theory are essential concepts in chemistry, and their connection provides a rich framework for understanding the world of chemistry.
Gallery of Ionic Compounds






FAQs
What is the NaCl model?
+The NaCl model, also known as the sodium chloride model, is a simple ionic model that describes the structure of sodium chloride (NaCl), commonly known as table salt.
What is Bohr's atomic theory?
+Bohr's atomic theory, introduced in 1913, is a concept that describes the structure of atoms, proposing that electrons occupy specific energy levels or shells around the nucleus.
How are the NaCl model and Bohr's atomic theory related?
+The NaCl model and Bohr's atomic theory are related through the concept of ionic bonding, where the electrostatic attraction between oppositely charged ions leads to the formation of a strong chemical bond.