Sodium, a naturally occurring element, is composed of multiple isotopes. These isotopes are variations of the same element with different numbers of neutrons in their atomic nuclei. Understanding the concept of isotopes is essential in various fields, including chemistry, physics, and earth sciences. In this article, we will delve into the world of sodium isotopes, exploring their properties, abundance, and significance.
Sodium Isotopes: What Are They?

Sodium isotopes are atoms of the same element with the same number of protons (11) in their atomic nuclei but differ in the number of neutrons. This variation in neutron number leads to differences in the atomic mass of the isotopes. The most common sodium isotopes are sodium-22, sodium-23, and sodium-24.
Properties of Sodium Isotopes
The properties of sodium isotopes are influenced by their atomic mass and neutron number. Here are some key properties of the most common sodium isotopes:
- Sodium-22 (Na-22): This isotope has 11 protons and 11 neutrons in its atomic nucleus. It is a radioactive isotope with a half-life of approximately 2.6 years.
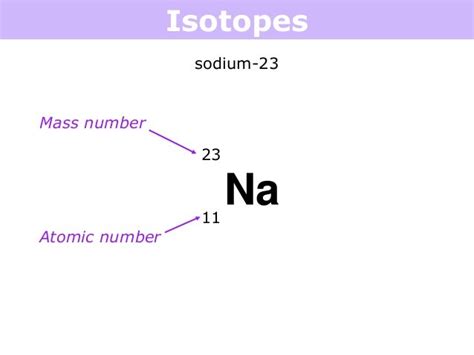
- Sodium-23 (Na-23): This isotope has 11 protons and 12 neutrons in its atomic nucleus. It is a stable isotope, making up about 99.3% of naturally occurring sodium.
- Sodium-24 (Na-24): This isotope has 11 protons and 13 neutrons in its atomic nucleus. It is a radioactive isotope with a half-life of approximately 14.96 hours.
Abundance of Sodium Isotopes

The abundance of sodium isotopes varies depending on their stability and the processes that produce them. Sodium-23 is the most abundant isotope, making up about 99.3% of naturally occurring sodium. Sodium-22 and sodium-24 are much less abundant, with concentrations of about 0.01% and 0.001%, respectively.
Significance of Sodium Isotopes
Sodium isotopes have various applications in science and industry. Here are some examples:
- Geology: Sodium isotopes are used in geology to study the movement of fluids in the Earth's crust and to date rocks.
- Medicine: Sodium-24 is used in nuclear medicine to study the distribution of sodium in the body and to diagnose certain medical conditions.
- Food Industry: Sodium-24 is used in the food industry to study the movement of sodium in food products and to develop new food processing technologies.
Conclusion
Sodium isotopes are naturally occurring variations of the element sodium, differing in the number of neutrons in their atomic nuclei. Understanding the properties, abundance, and significance of these isotopes is essential in various fields, including chemistry, physics, and earth sciences. By studying sodium isotopes, scientists can gain insights into the behavior of this important element and develop new applications for its use.
Practical Applications of Sodium Isotopes
- Tracing Sodium Movement: Sodium isotopes can be used to study the movement of sodium in various systems, such as the Earth's crust, the human body, and food products.
- Dating Rocks: Sodium isotopes can be used to date rocks and study the geological history of an area.
- Developing New Technologies: Sodium isotopes can be used to develop new technologies, such as new food processing methods and medical imaging techniques.
Gallery of Sodium Isotopes






What are sodium isotopes?
+Sodium isotopes are atoms of the same element with the same number of protons (11) in their atomic nuclei but differ in the number of neutrons.
What is the most abundant sodium isotope?
+The most abundant sodium isotope is sodium-23, making up about 99.3% of naturally occurring sodium.
What are some practical applications of sodium isotopes?
+Sodium isotopes can be used to study the movement of sodium in various systems, date rocks, and develop new technologies.
We hope this article has provided you with a comprehensive understanding of sodium isotopes and their significance. If you have any questions or would like to learn more about this topic, please feel free to ask in the comments section below.