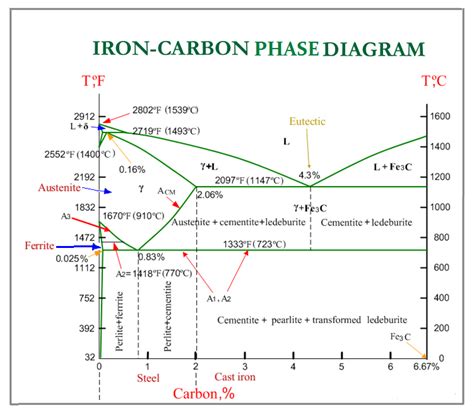
The iron-carbon phase equilibrium diagram is a fundamental tool in metallurgy and materials science, providing a comprehensive understanding of the behavior of iron-carbon alloys. This diagram is a graphical representation of the equilibrium states of iron-carbon alloys as a function of temperature and composition. In this article, we will delve into the details of the iron-carbon phase equilibrium diagram, exploring its significance, key features, and practical applications.
Importance of the Iron-Carbon Phase Equilibrium Diagram
The iron-carbon phase equilibrium diagram is crucial in understanding the microstructure and properties of steel and cast iron. Steel, which is a alloy of iron and carbon, is one of the most widely used materials in the world. The diagram helps metallurgists and materials scientists to predict the microstructure and properties of steel and cast iron, enabling them to design and develop new alloys with specific properties.

Key Features of the Iron-Carbon Phase Equilibrium Diagram
The iron-carbon phase equilibrium diagram is a complex diagram that shows the equilibrium states of iron-carbon alloys as a function of temperature and composition. The diagram consists of several key features, including:
- Austenite: Austenite is a face-centered cubic (FCC) crystal structure that is stable above the eutectoid temperature (727°C). Austenite is a single-phase region that exists in the diagram.
- Ferrite: Ferrite is a body-centered cubic (BCC) crystal structure that is stable below the eutectoid temperature (727°C). Ferrite is a single-phase region that exists in the diagram.
- Cementite: Cementite is a hard, brittle compound that is composed of iron and carbon. Cementite is a single-phase region that exists in the diagram.
- Pearlite: Pearlite is a two-phase region that consists of alternating layers of ferrite and cementite. Pearlite is formed when austenite is cooled slowly below the eutectoid temperature.
- Eutectoid Reaction: The eutectoid reaction is a three-phase reaction that occurs at the eutectoid temperature (727°C). The reaction involves the transformation of austenite to pearlite.
Practical Applications of the Iron-Carbon Phase Equilibrium Diagram
The iron-carbon phase equilibrium diagram has several practical applications in metallurgy and materials science. Some of the key applications include:
- Steel Production: The diagram is used to predict the microstructure and properties of steel, enabling steel producers to design and develop new steel alloys with specific properties.
- Heat Treatment: The diagram is used to predict the outcome of heat treatment processes, such as annealing, hardening, and tempering.
- Materials Selection: The diagram is used to select materials for specific applications, such as engine components, gears, and bearings.
Understanding the Iron-Carbon Phase Equilibrium Diagram
To understand the iron-carbon phase equilibrium diagram, it is essential to comprehend the underlying thermodynamic principles. The diagram is based on the Gibbs free energy of the iron-carbon system, which is a measure of the energy associated with the formation of a phase.

Interpretation of the Iron-Carbon Phase Equilibrium Diagram
The iron-carbon phase equilibrium diagram can be interpreted in several ways, depending on the specific application. Some of the key interpretations include:
- Isothermal Transformation: The diagram can be used to predict the outcome of isothermal transformation processes, such as the formation of pearlite.
- Continuous Cooling Transformation: The diagram can be used to predict the outcome of continuous cooling transformation processes, such as the formation of martensite.
- Thermal Analysis: The diagram can be used to predict the thermal analysis of iron-carbon alloys, such as the determination of the eutectoid temperature.
Gallery of Iron-Carbon Phase Equilibrium Diagram






What is the iron-carbon phase equilibrium diagram?
+The iron-carbon phase equilibrium diagram is a graphical representation of the equilibrium states of iron-carbon alloys as a function of temperature and composition.
What is the significance of the iron-carbon phase equilibrium diagram?
+The iron-carbon phase equilibrium diagram is crucial in understanding the microstructure and properties of steel and cast iron, enabling metallurgists and materials scientists to design and develop new alloys with specific properties.
What are the key features of the iron-carbon phase equilibrium diagram?
+The iron-carbon phase equilibrium diagram consists of several key features, including austenite, ferrite, cementite, pearlite, and the eutectoid reaction.
We hope this article has provided a comprehensive understanding of the iron-carbon phase equilibrium diagram and its significance in metallurgy and materials science. If you have any further questions or would like to discuss the topic in more detail, please leave a comment below.