Water, composed of hydrogen and oxygen atoms, is a substance that is essential for life as we know it. The chemical formula for water is H2O, which means it consists of two hydrogen atoms bonded to a single oxygen atom. However, the nature of the bond between these atoms is a topic of interest in chemistry. Is H2O ionic or covalent? In this article, we'll delve into the details of chemical bonding and explore the answer to this question.
The Basics of Chemical Bonding
Chemical bonding is the process by which atoms share or exchange electrons to form a chemical compound. There are several types of chemical bonds, but the two most relevant to our discussion are ionic and covalent bonds.
Ionic bonds are formed when one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges. The electrostatic attraction between these ions holds them together and forms a chemical compound. Typically, ionic bonds occur between metals and nonmetals.
Covalent bonds, on the other hand, are formed when two or more atoms share one or more pairs of electrons to achieve a stable electronic configuration. This type of bonding typically occurs between nonmetals.
The Nature of the Bond in H2O
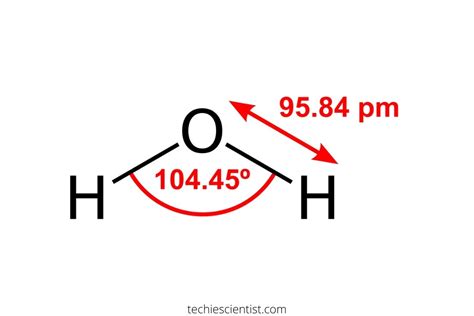
Now that we have a basic understanding of ionic and covalent bonds, let's examine the bond in H2O. The oxygen atom in H2O has six valence electrons, while each hydrogen atom has one valence electron. To achieve a stable electronic configuration, the oxygen atom shares its six valence electrons with the two hydrogen atoms, forming a covalent bond.

In this covalent bond, the oxygen atom shares two pairs of electrons with the two hydrogen atoms, forming a bent or V-shape molecular geometry. This sharing of electrons results in a polar covalent bond, meaning that the electrons are not shared equally between the atoms. The oxygen atom has a slightly negative charge, while the hydrogen atoms have a slightly positive charge.
Why H2O is Not Ionic
While it may seem that the transfer of electrons from the hydrogen atoms to the oxygen atom would result in an ionic bond, the reality is that the electrons are shared, not transferred. This sharing of electrons is a characteristic of covalent bonds, not ionic bonds.
Furthermore, the difference in electronegativity between oxygen and hydrogen is not sufficient to result in the formation of ions. The electronegativity of oxygen is 3.44, while that of hydrogen is 2.20. While there is a difference in electronegativity, it is not enough to result in the transfer of electrons and the formation of ions.
The Verdict
In conclusion, the bond in H2O is covalent, not ionic. The sharing of electrons between the oxygen and hydrogen atoms results in a polar covalent bond, which is a characteristic of covalent bonds. While there may be some confusion about the nature of the bond in H2O, the evidence clearly supports the conclusion that it is a covalent bond.
Key Characteristics of Covalent Bonds in H2O
- Sharing of electrons between atoms
- Polar covalent bond due to unequal sharing of electrons
- Bent or V-shape molecular geometry
- Oxygen atom has a slightly negative charge, while hydrogen atoms have a slightly positive charge
Comparison of Ionic and Covalent Bonds
| Ionic Bonds | Covalent Bonds | |
|---|---|---|
| Type of Bond | Formed by transfer of electrons | Formed by sharing of electrons |
| Atoms Involved | Typically between metals and nonmetals | Typically between nonmetals |
| Electron Configuration | Ions with opposite charges | Shared electrons result in a stable electronic configuration |
| Examples | NaCl, CaO | H2O, CO2, CH4 |

Gallery of Covalent Bonds in H2O






What type of bond is formed in H2O?
+The bond in H2O is a covalent bond, specifically a polar covalent bond.
Why is the bond in H2O not ionic?
+The bond in H2O is not ionic because the electrons are shared, not transferred, between the oxygen and hydrogen atoms.
What is the molecular geometry of H2O?
+The molecular geometry of H2O is bent or V-shape.
In conclusion, the bond in H2O is a covalent bond, specifically a polar covalent bond. The sharing of electrons between the oxygen and hydrogen atoms results in a stable electronic configuration and a bent or V-shape molecular geometry. We hope this article has helped you understand the nature of the bond in H2O. If you have any further questions or comments, please don't hesitate to reach out.