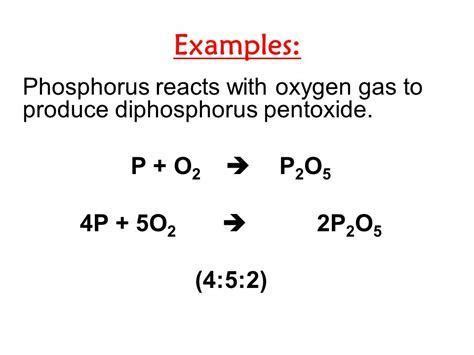
Diphosphorus pentoxide, commonly referred to as phosphorus pentoxide, is a fascinating compound that has numerous applications in various fields. Its unique properties and reactions make it an interesting subject to explore. In this article, we will delve into five key facts about the diphosphorus pentoxide formula, shedding light on its composition, structure, uses, and more.
What is Diphosphorus Pentoxide?

Diphosphorus pentoxide is a covalent compound with the chemical formula P4O10 or P2O5. It is composed of four phosphorus atoms and ten oxygen atoms, making it a highly oxidized molecule. The compound is a colorless, odorless, and tasteless solid that is highly reactive, especially when it comes into contact with water.
Structure and Properties of Diphosphorus Pentoxide

The diphosphorus pentoxide formula indicates that it is a molecular compound, consisting of discrete P4O10 molecules. Each phosphorus atom is bonded to three oxygen atoms, forming a tetrahedral arrangement. The compound has a high melting point of around 340°C and a boiling point of 423°C. It is also highly soluble in water, which is one of the reasons it is commonly used as a desiccant.
Uses of Diphosphorus Pentoxide
Diphosphorus pentoxide has a wide range of applications in various industries, including:
- Desiccant: Due to its high affinity for water, diphosphorus pentoxide is commonly used as a desiccant to remove moisture from gases and liquids.
- Catalyst: The compound is used as a catalyst in various chemical reactions, such as the synthesis of polyethylene and polypropylene.
- Food industry: Diphosphorus pentoxide is used as a food additive, helping to prevent spoilage and extend the shelf life of food products.
- Pharmaceuticals: The compound is used in the production of certain pharmaceuticals, such as painkillers and antacids.
Preparation and Synthesis of Diphosphorus Pentoxide

Diphosphorus pentoxide can be prepared through the combustion of white phosphorus in air. The reaction is highly exothermic, releasing a significant amount of heat and light. The compound can also be synthesized through the reaction of phosphorus trichloride with oxygen.
Handling and Safety Precautions
When handling diphosphorus pentoxide, it is essential to take necessary safety precautions to avoid exposure. The compound is highly reactive and can cause severe burns and respiratory problems. Some of the safety precautions include:
- Wearing protective gear: Wear gloves, goggles, and a face mask when handling the compound.
- Working in a well-ventilated area: Avoid inhaling the dust or fumes emitted by the compound.
- Storing in a dry environment: Keep the compound away from moisture and humidity.
Gallery of Phosphorus Pentoxide






We hope this article has provided you with a comprehensive understanding of the diphosphorus pentoxide formula and its properties. Whether you are a student, researcher, or industry professional, we encourage you to share your thoughts and insights on this fascinating compound.
What is the diphosphorus pentoxide formula?
+The diphosphorus pentoxide formula is P4O10 or P2O5.
What are the uses of diphosphorus pentoxide?
+Diphosphorus pentoxide is used as a desiccant, catalyst, food additive, and in the production of certain pharmaceuticals.
How is diphosphorus pentoxide prepared?
+Diphosphorus pentoxide can be prepared through the combustion of white phosphorus in air or through the reaction of phosphorus trichloride with oxygen.