Understanding the GE Atom Lewis Structure is essential for grasping the fundamental concepts of chemistry, particularly when it comes to the behavior of atoms and their interactions. In this article, we will delve into the world of atomic structures, focusing on the GE Atom Lewis Structure, and explain it in a simple and concise manner.
What is the GE Atom Lewis Structure?
The GE Atom Lewis Structure, also known as the Lewis Structure or Electron Dot Structure, is a graphical representation of the arrangement of electrons in an atom. This structure was first introduced by Gilbert N. Lewis in 1916 and is widely used to visualize the valence electrons of an atom. The GE Atom Lewis Structure is particularly useful for understanding the chemical bonding between atoms and predicting the properties of molecules.
Why is the GE Atom Lewis Structure Important?
The GE Atom Lewis Structure is crucial in understanding various chemical concepts, including:
- Chemical Bonding: The Lewis Structure helps predict the type of bond that will form between atoms, whether it's ionic, covalent, or metallic.
- Molecular Shape: The arrangement of electrons in the Lewis Structure determines the shape of a molecule, which is essential for understanding its physical and chemical properties.
- Reactivity: The Lewis Structure provides insight into an atom's reactivity, including its ability to form bonds and participate in chemical reactions.
How to Draw the GE Atom Lewis Structure
Drawing the GE Atom Lewis Structure involves a few simple steps:
- Determine the Number of Valence Electrons: Identify the number of valence electrons in the atom, which is typically the number of electrons in the outermost energy level.
- Draw the Central Atom: Draw the central atom, which is the atom that will be surrounded by other atoms.
- Add Electrons: Add the valence electrons to the central atom, using dots (•) to represent each electron.
- Form Bonds: Use lines to represent bonds between atoms, with each line representing a pair of shared electrons.
- Satisfy the Octet Rule: Ensure that each atom has a full outer energy level, with eight electrons in the valence shell, unless it's a noble gas.
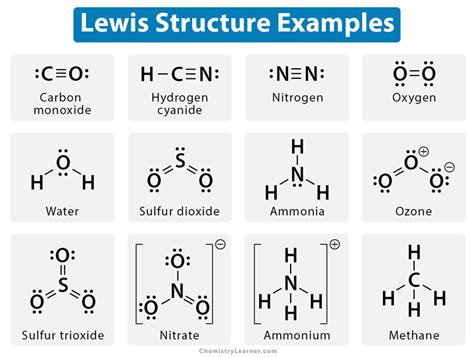
Examples of GE Atom Lewis Structures
Let's consider a few examples to illustrate the GE Atom Lewis Structure:
- Hydrogen (H): The Lewis Structure for hydrogen consists of a single electron dot (•) representing the single valence electron.
- Oxygen (O): The Lewis Structure for oxygen consists of six electron dots (• • • • • •) representing the six valence electrons.
- Carbon (C): The Lewis Structure for carbon consists of four electron dots (• • • •) representing the four valence electrons.

Benefits of the GE Atom Lewis Structure
The GE Atom Lewis Structure offers several benefits, including:
- Simplified Understanding: The Lewis Structure provides a simple and visual representation of atomic structures, making it easier to understand complex chemical concepts.
- Predictive Power: The Lewis Structure allows chemists to predict the properties and behavior of molecules, including their reactivity and shape.
- Improved Communication: The Lewis Structure provides a common language for chemists to communicate and share their understanding of atomic structures.
Common Misconceptions
There are several common misconceptions about the GE Atom Lewis Structure:
- Misconception 1: The Lewis Structure is not a physical representation of the atom, but rather a graphical representation of the arrangement of electrons.
- Misconception 2: The Lewis Structure is not limited to simple molecules, but can be applied to complex molecules and ions.
Gallery of GE Atom Lewis Structures






Frequently Asked Questions
What is the purpose of the GE Atom Lewis Structure?
+The GE Atom Lewis Structure is used to visualize the arrangement of electrons in an atom, which helps predict the properties and behavior of molecules.
How do I draw the GE Atom Lewis Structure?
+To draw the GE Atom Lewis Structure, determine the number of valence electrons, draw the central atom, add electrons, form bonds, and satisfy the octet rule.
What are the benefits of the GE Atom Lewis Structure?
+The GE Atom Lewis Structure provides a simplified understanding of atomic structures, predictive power, and improved communication among chemists.
In conclusion, the GE Atom Lewis Structure is a powerful tool for understanding the arrangement of electrons in atoms and predicting the properties and behavior of molecules. By following the simple steps outlined in this article, you can draw the GE Atom Lewis Structure for any atom and gain a deeper understanding of the fundamental concepts of chemistry.