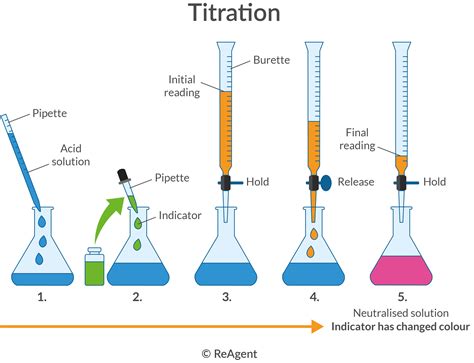
Titration, a laboratory technique used to determine the concentration of a substance, has numerous practical applications in various fields. From quality control in manufacturing to medical diagnosis, titration plays a crucial role in ensuring accuracy and precision. In this article, we will explore the real-life applications and uses of titration, highlighting its importance in different industries.
What is Titration?
Titration is a laboratory technique used to determine the concentration of a substance by reacting it with a known amount of another substance. The reaction is usually a neutralization reaction, where an acid and a base react to form a salt and water. The endpoint of the reaction is indicated by a color change, which signals the completion of the reaction. By measuring the amount of substance required to reach the endpoint, the concentration of the unknown substance can be calculated.
Quality Control in Manufacturing
Titration is widely used in quality control processes in manufacturing to ensure the quality of raw materials and finished products. In the food industry, titration is used to determine the acidity of food products, such as fruit juices and wines. In the pharmaceutical industry, titration is used to determine the concentration of active ingredients in medications.
For example, in the production of food products, titration is used to determine the pH level of the product. If the pH level is too high or too low, it can affect the texture, flavor, and shelf life of the product. By using titration to adjust the pH level, manufacturers can ensure that their products meet the required standards.

Medical Diagnosis
Titration is used in medical diagnosis to determine the concentration of certain substances in the body. For example, in the diagnosis of diabetes, titration is used to measure the concentration of glucose in the blood. By measuring the amount of glucose in the blood, doctors can determine whether a patient has diabetes or not.
In addition, titration is used to determine the concentration of certain medications in the blood. This is particularly useful in monitoring the effectiveness of medications and adjusting dosages accordingly.
Environmental Monitoring
Titration is used in environmental monitoring to determine the concentration of pollutants in water and air. For example, in the monitoring of water quality, titration is used to determine the concentration of heavy metals, such as lead and mercury. By measuring the concentration of these pollutants, environmental scientists can determine whether the water is safe for human consumption.
In addition, titration is used to monitor the concentration of greenhouse gases, such as carbon dioxide and methane. By measuring the concentration of these gases, scientists can determine the impact of human activities on the environment.
Forensic Analysis
Titration is used in forensic analysis to determine the concentration of certain substances in evidence samples. For example, in the analysis of blood samples, titration is used to measure the concentration of alcohol. By measuring the amount of alcohol in the blood, forensic scientists can determine whether a person was intoxicated at the time of a crime.
In addition, titration is used to analyze the concentration of certain substances in hair and nail samples. By measuring the concentration of these substances, forensic scientists can determine whether a person has been exposed to certain substances, such as drugs or poisons.
Pharmaceutical Development
Titration is used in pharmaceutical development to determine the concentration of active ingredients in medications. By measuring the concentration of active ingredients, pharmaceutical companies can ensure that their medications meet the required standards.
In addition, titration is used to develop new medications. By measuring the concentration of active ingredients, pharmaceutical companies can determine the effectiveness of new medications and adjust dosages accordingly.
Food and Beverage Industry
Titration is used in the food and beverage industry to determine the concentration of certain substances in food products. For example, in the production of fruit juices, titration is used to determine the concentration of acidity. By measuring the concentration of acidity, manufacturers can ensure that their products meet the required standards.
In addition, titration is used to determine the concentration of certain substances in beverages, such as coffee and tea. By measuring the concentration of these substances, manufacturers can ensure that their products meet the required standards.
Gallery of Titration in Real Life





FAQs
What is titration?
+Titration is a laboratory technique used to determine the concentration of a substance by reacting it with a known amount of another substance.
What are the applications of titration?
+Titration has numerous applications in various fields, including quality control in manufacturing, medical diagnosis, environmental monitoring, forensic analysis, and pharmaceutical development.
How is titration used in quality control?
+Titration is used in quality control to determine the concentration of certain substances in raw materials and finished products, ensuring that they meet the required standards.
In conclusion, titration is a versatile laboratory technique with numerous practical applications in various fields. From quality control in manufacturing to medical diagnosis, titration plays a crucial role in ensuring accuracy and precision. By understanding the principles and applications of titration, we can appreciate its importance in our daily lives.