Germanium, a metalloid element with the symbol Ge, is a fascinating subject in the realm of chemistry. Understanding its atomic structure is crucial for grasping its properties and behavior. In this article, we will delve into the world of Germanium and explore its atomic structure, specifically focusing on the Ge atom Lewis structure.
What is Germanium?
Before diving into the Lewis structure of Germanium, let's briefly discuss what it is. Germanium is a chemical element with the atomic number 32 and the symbol Ge. It is a metalloid, meaning it exhibits some properties of metals and some properties of nonmetals. Germanium is a hard, gray-white, brittle metalloid that is used in a wide range of applications, including electronics, solar cells, and fiber optics.

What is a Lewis Structure?
A Lewis structure, also known as a Lewis dot structure, is a graphical representation of the valence electrons of an atom. It is a simple and convenient way to visualize the arrangement of electrons in an atom and to predict the chemical properties of an element. Lewis structures are composed of dots, which represent valence electrons, and lines, which represent chemical bonds.
How to Draw a Lewis Structure
Drawing a Lewis structure involves several steps:
- Determine the total number of valence electrons in the atom.
- Draw the symbol of the atom.
- Place the valence electrons around the atom, following the octet rule.
- Connect the electrons with lines to form chemical bonds.
Ge Atom Lewis Structure
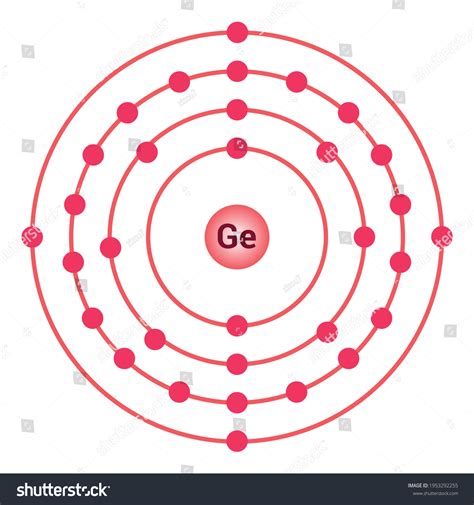
Now that we have a basic understanding of Lewis structures, let's dive into the Ge atom Lewis structure. Germanium has an atomic number of 32, which means it has 32 protons and 32 electrons. The electron configuration of Germanium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p²
To draw the Lewis structure of Germanium, we need to focus on the valence electrons, which are the electrons in the outermost energy level. The valence electrons of Germanium are:
4s² 4p²
Here is the Lewis structure of Germanium:

As you can see, the Lewis structure of Germanium consists of four valence electrons, which are arranged in a tetrahedral shape. This shape is due to the fact that Germanium has four bonding pairs of electrons, which are represented by the four lines.
Properties of Germanium
The Lewis structure of Germanium can help us understand some of its properties. For example:
- Germanium is a metalloid, which means it exhibits some properties of metals and some properties of nonmetals. This is reflected in its Lewis structure, which shows a mix of metal-like and nonmetal-like bonding.
- Germanium has a relatively high melting point and boiling point, which is due to the strong bonds between its atoms.
- Germanium is a semiconductor, which means it can conduct electricity under certain conditions. This is due to the fact that its Lewis structure shows a mix of filled and empty orbitals, which allows it to conduct electricity.
Gallery of Germanium






Frequently Asked Questions
What is the atomic number of Germanium?
+The atomic number of Germanium is 32.
What is the electron configuration of Germanium?
+The electron configuration of Germanium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p².
What is the Lewis structure of Germanium?
+The Lewis structure of Germanium consists of four valence electrons, which are arranged in a tetrahedral shape.
In conclusion, understanding the Ge atom Lewis structure is crucial for grasping the properties and behavior of Germanium. The Lewis structure shows a mix of metal-like and nonmetal-like bonding, which reflects Germanium's metalloid nature. By studying the Lewis structure of Germanium, we can gain insights into its electronic configuration, chemical properties, and physical properties.