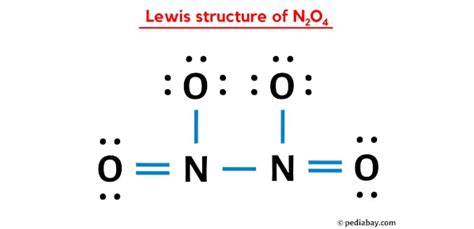
Drawing the Lewis structure of N2O4 can be a challenging task, but with the right steps, it can be accomplished easily. In this article, we will guide you through the process of drawing the N2O4 Lewis structure in 4 easy steps.
Understanding the Basics
Before we dive into the steps, let's quickly review the basics of Lewis structures. A Lewis structure is a representation of the valence electrons in a molecule using dots and lines. It's a way to visualize the bonding and structure of a molecule. To draw a Lewis structure, we need to know the molecular formula of the compound, the number of valence electrons, and the octet rule.
What is N2O4?
N2O4, also known as dinitrogen tetroxide, is a chemical compound composed of two nitrogen atoms and four oxygen atoms. It's a colorless liquid with a characteristic sweet, pungent odor. N2O4 is used in various industrial applications, including the production of nitric acid and rocket propellants.
Step 1: Determine the Total Number of Valence Electrons
To draw the Lewis structure of N2O4, we need to determine the total number of valence electrons. Nitrogen has 5 valence electrons, and oxygen has 6 valence electrons. Since there are two nitrogen atoms and four oxygen atoms, the total number of valence electrons is:
2 x 5 (nitrogen) + 4 x 6 (oxygen) = 10 + 24 = 34 valence electrons
Step 2: Draw the Skeleton Structure
Next, we draw the skeleton structure of N2O4, which is the arrangement of atoms in the molecule. The nitrogen atoms are bonded to each other, and each nitrogen atom is bonded to two oxygen atoms.

Step 3: Add Electrons to the Skeleton Structure
Now, we add electrons to the skeleton structure to satisfy the octet rule. We start by adding electrons to the nitrogen atoms, which need 8 electrons to satisfy the octet rule. We then add electrons to the oxygen atoms, which also need 8 electrons.

Step 4: Finalize the Lewis Structure
Finally, we finalize the Lewis structure by ensuring that all atoms have a full octet and that the molecule is neutral. We can see that the nitrogen atoms have 8 electrons, and the oxygen atoms have 8 electrons.

Gallery of N2O4 Lewis Structure



Frequently Asked Questions
What is the molecular formula of N2O4?
+The molecular formula of N2O4 is N2O4.
How many valence electrons does N2O4 have?
+N2O4 has 34 valence electrons.
What is the octet rule in chemistry?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons.
By following these 4 easy steps, you can draw the Lewis structure of N2O4. Remember to always follow the octet rule and ensure that all atoms have a full octet. Practice drawing Lewis structures to become more comfortable with the process.