The world of chemistry is a vast and fascinating place, full of intricate reactions and processes that shape the very fabric of our universe. Among the many elements that make up this complex tapestry, sodium and chlorine hold a special significance, particularly when it comes to their bonding properties. In this article, we will delve into the fascinating world of sodium and chlorine bonding, exploring the chemistry that underlies their unique relationship.
What is Sodium?
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is the sixth most abundant element in the Earth's crust. Sodium is highly reactive, readily losing one electron to form a positive ion (Na+). This property makes it highly useful in a wide range of applications, from the production of soap and paper to the manufacture of pharmaceuticals.

What is Chlorine?
Chlorine is a chemical element with the symbol Cl and atomic number 17. It is a yellow-green gas at room temperature and is highly reactive, readily forming compounds with other elements. Chlorine is a member of the halogen family, which also includes fluorine, bromine, and iodine. Chlorine is widely used in a variety of applications, including the production of disinfectants, sanitizers, and plastics.

The Bonding of Sodium and Chlorine
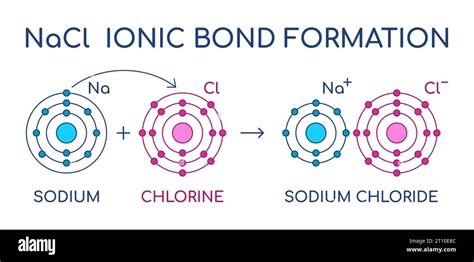
Sodium and chlorine form a highly stable compound known as sodium chloride, or common table salt (NaCl). This compound is formed through a process known as ionic bonding, in which the highly reactive sodium atom loses an electron to form a positive ion (Na+), while the chlorine atom gains an electron to form a negative ion (Cl-). The electrostatic attraction between the positively charged sodium ion and the negatively charged chlorine ion holds the compound together, forming a highly stable crystal lattice structure.

How Does Ionic Bonding Work?
Ionic bonding is a type of chemical bonding that involves the transfer of electrons between atoms. In the case of sodium and chlorine, the highly reactive sodium atom readily loses an electron to form a positive ion (Na+), while the chlorine atom gains an electron to form a negative ion (Cl-). The electrostatic attraction between the positively charged sodium ion and the negatively charged chlorine ion holds the compound together, forming a highly stable crystal lattice structure.
Properties of Sodium Chloride
Sodium chloride is a highly stable compound with a number of unique properties. It is highly soluble in water, forming a clear, colorless solution. It is also highly stable, with a melting point of 801°C and a boiling point of 1413°C. Sodium chloride is also highly conductive, able to conduct electricity when dissolved in water.

Uses of Sodium Chloride
Sodium chloride is widely used in a variety of applications, including:
- Food preservation: Sodium chloride is used as a preservative in a wide range of food products, including meats, vegetables, and soups.
- Medical applications: Sodium chloride is used in medical applications, including the production of saline solutions for IV drips and eye drops.
- Industrial applications: Sodium chloride is used in a variety of industrial applications, including the production of soap, paper, and plastics.
Gallery of Sodium Chloride





What is sodium chloride?
+Sodium chloride is a chemical compound composed of sodium and chlorine atoms, commonly known as table salt.
What is the structure of sodium chloride?
+Sodium chloride has a crystalline structure, with positively charged sodium ions and negatively charged chloride ions arranged in a lattice pattern.
What are some common uses of sodium chloride?
+Sodium chloride is widely used as a preservative in food products, in medical applications, and in industrial processes.
In conclusion, the bonding of sodium and chlorine is a complex and fascinating process that underlies the unique properties of sodium chloride. By understanding the chemistry of this compound, we can appreciate the importance of sodium chloride in a wide range of applications, from food preservation to medical treatments. Whether you are a scientist, a cook, or simply someone interested in the natural world, the story of sodium and chlorine bonding is sure to captivate and inspire.