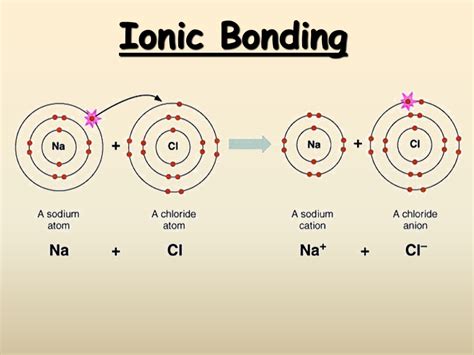
Ionic bonds are a type of chemical bond that forms between two atoms that have a large difference in electronegativity. This type of bond is typically found in compounds that consist of a metal and a nonmetal. In this article, we will explore the concept of ionic bonds, specifically the bond between potassium (K) and chlorine (Cl), and delve into the intricacies of their interaction.
What is an Ionic Bond?
An ionic bond is a type of chemical bond that forms between two atoms that have a large difference in electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself. When an atom with high electronegativity, such as a nonmetal, is paired with an atom that has low electronegativity, such as a metal, the electrons are transferred from the metal atom to the nonmetal atom. This transfer of electrons creates a positive ion (cation) and a negative ion (anion), which are then attracted to each other, forming an ionic bond.

Formation of KCl
Potassium (K) is a highly reactive metal that has a low electronegativity value of 0.82. Chlorine (Cl), on the other hand, is a highly reactive nonmetal that has a high electronegativity value of 3.16. When potassium and chlorine are combined, the electrons from the potassium atom are transferred to the chlorine atom, creating a positive potassium ion (K+) and a negative chloride ion (Cl-).

The resulting compound, potassium chloride (KCl), is a highly stable ionic compound that is commonly used as a salt substitute and in various industrial applications.
Properties of KCl
KCl has several distinct properties that are characteristic of ionic compounds. Some of these properties include:
- High melting point: KCl has a melting point of 770°C, which is typical of ionic compounds.
- High boiling point: KCl has a boiling point of 1413°C, which is also typical of ionic compounds.
- Solubility: KCl is highly soluble in water, which is characteristic of ionic compounds.
- Conductivity: KCl is a good conductor of electricity when dissolved in water, which is also characteristic of ionic compounds.

Conclusion
In conclusion, the bond between potassium (K) and chlorine (Cl) is a classic example of an ionic bond. The large difference in electronegativity between the two atoms creates a strong electrostatic attraction, resulting in the formation of a highly stable ionic compound. Understanding the properties and behavior of ionic compounds like KCl is essential in various fields, including chemistry, physics, and materials science.





What is an ionic bond?
+An ionic bond is a type of chemical bond that forms between two atoms that have a large difference in electronegativity.
What is the difference between a metal and a nonmetal?
+A metal is an element that has a low electronegativity value, while a nonmetal is an element that has a high electronegativity value.
What is the resulting compound when potassium and chlorine are combined?
+The resulting compound is potassium chloride (KCl), a highly stable ionic compound.