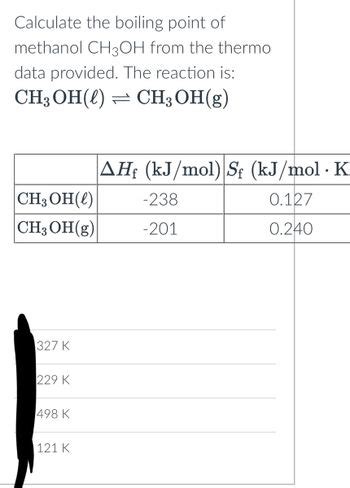
The boiling point of CH3OH, also known as methanol, is 64.7°C (148.5°F) at standard atmospheric pressure.
Methanol is a polar solvent with a relatively high boiling point due to the strong hydrogen bonding between its molecules. The boiling point of methanol is higher than that of many other organic solvents, such as ethanol (78.3°C) and acetone (56.3°C), but lower than that of water (100°C).
It's worth noting that the boiling point of methanol can vary slightly depending on the purity of the sample and the presence of impurities. However, 64.7°C is the accepted boiling point of pure methanol at standard atmospheric pressure.
Here is the image for Methanol Boiling Point

Properties of Methanol
Methanol is a colorless, volatile liquid with a characteristic odor. It is highly flammable and has a flash point of 11°C (52°F). Methanol is also highly toxic and can cause serious health effects if ingested or inhaled.
Uses of Methanol
Methanol has a wide range of applications in various industries, including:
- Fuel: Methanol is used as a fuel in internal combustion engines, particularly in racing cars.
- Solvent: Methanol is used as a solvent in the production of various chemicals, such as formaldehyde and acetic acid.
- Antifreeze: Methanol is used as an antifreeze in windshield washing fluids and other applications.
- Pharmaceuticals: Methanol is used as a solvent and intermediate in the production of certain pharmaceuticals.
Overall, methanol is an important chemical with a wide range of applications, and its boiling point is an important property that is relevant to its use and handling.
Gallery of Methanol






FAQs
What is the boiling point of methanol?
+The boiling point of methanol is 64.7°C (148.5°F) at standard atmospheric pressure.
What are the uses of methanol?
+Methanol is used as a fuel, solvent, antifreeze, and intermediate in the production of various chemicals and pharmaceuticals.
Is methanol toxic?
+Yes, methanol is highly toxic and can cause serious health effects if ingested or inhaled.