The world of chemistry is full of fascinating concepts that help us understand the behavior of atoms and molecules. One such concept is the transfer of valence electrons, which plays a crucial role in the formation of chemical bonds. In this article, we will delve into the world of valence electrons and explore how their transfer leads to the creation of ions and molecules.

What are Valence Electrons?
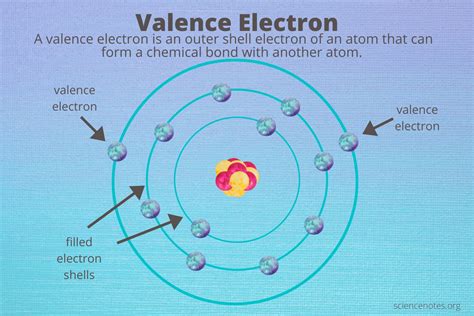
Valence electrons are the electrons in an atom that participate in chemical bonding. They are the outermost electrons of an atom, located in the valence shell. The valence shell is the outermost energy level of an atom, and it is where the electrons that are involved in chemical bonding are found.
The number of valence electrons in an atom determines its chemical properties. Atoms with a full valence shell are stable and do not readily form chemical bonds. Atoms with an incomplete valence shell, on the other hand, are reactive and tend to form chemical bonds with other atoms to achieve a full valence shell.
Types of Valence Electrons
There are two types of valence electrons: s-electrons and p-electrons. S-electrons are found in the s-orbitals, which are spherical in shape. P-electrons are found in the p-orbitals, which are dumbbell-shaped.

How Valence Electrons Transfer
Valence electrons transfer occurs when an atom gains or loses electrons to form ions. When an atom loses one or more valence electrons, it becomes a positively charged ion, known as a cation. When an atom gains one or more valence electrons, it becomes a negatively charged ion, known as an anion.
The transfer of valence electrons is a key concept in understanding chemical bonding. It is the transfer of valence electrons that leads to the formation of ions, which are the building blocks of molecules.
Ionic Bonding
Ionic bonding is a type of chemical bonding that involves the transfer of valence electrons between atoms. It is the electrostatic attraction between oppositely charged ions that holds them together.
Ionic bonds are typically formed between metals and nonmetals. Metals tend to lose electrons to form cations, while nonmetals tend to gain electrons to form anions.

Examples of Ionic Bonding
- Sodium chloride (NaCl): Sodium (Na) loses an electron to form a cation, while chlorine (Cl) gains an electron to form an anion. The electrostatic attraction between the oppositely charged ions holds them together.
- Calcium carbonate (CaCO3): Calcium (Ca) loses two electrons to form a cation, while carbonate (CO3) gains two electrons to form an anion. The electrostatic attraction between the oppositely charged ions holds them together.
Covalent Bonding
Covalent bonding is a type of chemical bonding that involves the sharing of valence electrons between atoms. It is the sharing of electrons that holds the atoms together.
Covalent bonds are typically formed between nonmetals. Nonmetals tend to share electrons to form molecules.

Examples of Covalent Bonding
- Hydrogen gas (H2): Two hydrogen atoms share their valence electrons to form a covalent bond.
- Oxygen gas (O2): Two oxygen atoms share their valence electrons to form a covalent bond.
Gallery of Valence Electrons Transfer






FAQs
What are valence electrons?
+Valence electrons are the electrons in an atom that participate in chemical bonding.
How do valence electrons transfer?
+Valence electrons transfer occurs when an atom gains or loses electrons to form ions.
What is ionic bonding?
+Ionic bonding is a type of chemical bonding that involves the transfer of valence electrons between atoms.
We hope this article has helped you understand the concept of valence electrons transfer and its role in chemical bonding. Whether you're a student or a professional, understanding valence electrons transfer is essential for grasping the fundamental principles of chemistry.