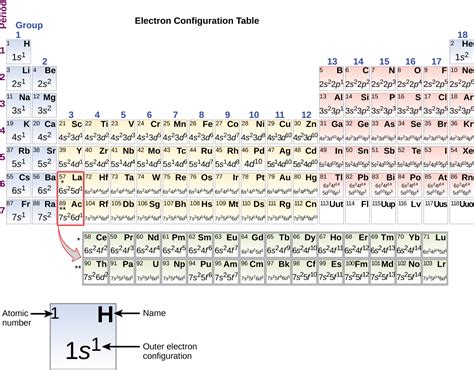
The atomic number of an element is equal to the number of protons in its atomic nucleus, and it determines the element's position in the periodic table. The number of electrons in a neutral atom is equal to the number of protons, and it is also equal to the atomic number.
An element with 17 electrons would have an atomic number of 17, which corresponds to the element Chlorine (Cl). Chlorine is a halogen and is located in group 17 of the periodic table.
Here is a brief overview of Chlorine:
Atomic Number: 17 Atomic Mass: 35.45 u (unified atomic mass units) Electron Configuration: [Ne] 3s² 3p⁵ Number of Electrons: 17 Number of Protons: 17 Number of Neutrons: 18 (in the most common isotope, Chlorine-35)
Chlorine is a yellow-green gas at room temperature and is highly reactive. It is widely used in various industries, including water treatment, paper bleaching, and the manufacture of plastics and textiles.

In summary, the element with 17 electrons is Chlorine, which has an atomic number of 17 and is located in group 17 of the periodic table.
Benefits of Knowing the Number of Electrons
Understanding the number of electrons in an atom is crucial in chemistry, as it helps us predict the element's properties and behavior. The number of electrons determines the element's reactivity, its ability to form bonds with other elements, and its position in the periodic table.
For example, Chlorine's 17 electrons make it highly reactive, as it needs to gain only one electron to complete its outer energy level and achieve a stable electron configuration. This is why Chlorine is often used as a disinfectant and sanitizer, as it can easily react with other substances to form compounds that are toxic to microorganisms.
How to Determine the Number of Electrons
To determine the number of electrons in an atom, you need to know the atomic number of the element. The atomic number is equal to the number of protons in the atomic nucleus, and it is also equal to the number of electrons in a neutral atom.
You can find the atomic number of an element by looking up its symbol in the periodic table or by using an online periodic table. Once you know the atomic number, you can determine the number of electrons by using the following formula:
Number of electrons = Atomic number
For example, if the atomic number of an element is 17, then the number of electrons is also 17.
Electron Configuration
The electron configuration of an atom is a way of describing the arrangement of electrons in the atom's energy levels or electron shells. The electron configuration is written in a specific notation, which includes the energy level, the subshell, and the number of electrons in each subshell.
For example, the electron configuration of Chlorine is:
[Ne] 3s² 3p⁵
This notation tells us that Chlorine has a full outer energy level (the [Ne] notation indicates a full outer energy level), and that the 3s subshell has two electrons and the 3p subshell has five electrons.

Gallery of Chlorine Compounds






Frequently Asked Questions
What is the atomic number of Chlorine?
+The atomic number of Chlorine is 17.
How many electrons does Chlorine have?
+Chlorine has 17 electrons.
What is the electron configuration of Chlorine?
+The electron configuration of Chlorine is [Ne] 3s² 3p⁵.
In conclusion, the element with 17 electrons is Chlorine, which has an atomic number of 17 and is located in group 17 of the periodic table. Understanding the number of electrons in an atom is crucial in chemistry, as it helps us predict the element's properties and behavior.