The concept of weight and volume is a fundamental aspect of physics, and understanding the relationship between these two physical properties can reveal fascinating insights into the nature of materials. In this article, we will explore the tungsten weight by volume, specifically examining the density of this element.
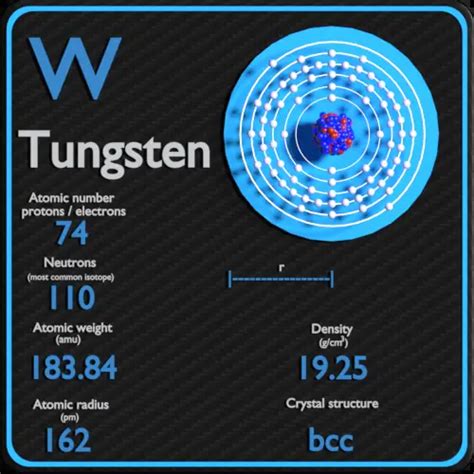
Tungsten, also known as wolfram, is a chemical element with the symbol W and atomic number 74. It is a hard, dense, gray-white to gray-blue transition metal that is highly valued for its unique properties. Tungsten has the highest melting point of all elements, with a boiling point of 3422°C (6192°F). This makes it an ideal material for high-temperature applications, such as filaments in incandescent light bulbs and rocket nozzles.
Understanding Density
Density is defined as the mass per unit volume of a substance. It is a measure of how much matter is packed into a given volume. Density is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). In the case of tungsten, its density is approximately 19.3 g/cm³, which is one of the highest among all elements.

Why is Tungsten So Dense?
So, why does tungsten have such a high density? The answer lies in its atomic structure. Tungsten has a hexagonal close-packed crystal structure, which means that its atoms are arranged in a repeating pattern of hexagons. This arrangement allows for a high packing efficiency, resulting in a dense material. Additionally, tungsten has a high atomic mass, with an atomic weight of 183.84 u (unified atomic mass units). This means that each tungsten atom contributes a significant amount of mass to the overall density of the material.
Calculating Tungsten Weight by Volume
Now that we understand the density of tungsten, let's explore how to calculate its weight by volume. The formula for density is:
density = mass / volume
Rearranging this formula to solve for mass, we get:
mass = density x volume
Given the density of tungsten (19.3 g/cm³), we can calculate the mass of a given volume of tungsten.

Example Calculation
Let's say we have a cube of tungsten with a side length of 1 cm. To calculate its mass, we need to first calculate its volume:
volume = side length³ = 1 cm³ = 1 cm x 1 cm x 1 cm
Using the density of tungsten, we can now calculate its mass:
mass = density x volume = 19.3 g/cm³ x 1 cm³ = 19.3 g
Therefore, a 1-cm³ cube of tungsten has a mass of approximately 19.3 grams.
Applications of Tungsten's High Density
The high density of tungsten makes it an ideal material for various applications. Some examples include:
-
High-Speed Cutting Tools
Tungsten's high density and hardness make it an ideal material for cutting tools, such as drill bits and saw blades. These tools can withstand high speeds and heavy loads, making them suitable for machining hard materials.

-
Shielding and Radiation Protection
Tungsten's high density and atomic number make it an effective material for shielding and radiation protection. It is often used in medical and industrial applications where radiation protection is critical.

Conclusion
In conclusion, the tungsten weight by volume is a fascinating topic that reveals the unique properties of this element. Its high density, resulting from its atomic structure and high atomic mass, makes it an ideal material for various applications. By understanding the density of tungsten, we can calculate its weight by volume and explore its potential uses in different fields.






What is the density of tungsten?
+The density of tungsten is approximately 19.3 g/cm³.
What is the atomic structure of tungsten?
+Tungsten has a hexagonal close-packed crystal structure.
What are some applications of tungsten's high density?
+Tungsten's high density makes it an ideal material for high-speed cutting tools, shielding, and radiation protection.