Water is essential for human life, and its boiling point is a fundamental physical property that has numerous applications in various fields, including science, engineering, and cooking. In this article, we will delve into the world of thermodynamics and explore the boiling point of water in Kelvin.
The Importance of Boiling Point
The boiling point of a substance is the temperature at which it changes state from a liquid to a gas. This property is crucial in understanding various physical and chemical processes, such as cooking, chemical reactions, and energy transfer. In the case of water, its boiling point is a critical factor in determining the quality of drinking water, the efficiency of steam engines, and the safety of industrial processes.
What is Kelvin?
Kelvin is a unit of temperature that is defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. In simpler terms, Kelvin is a temperature scale that is used to measure extremely low temperatures, typically in scientific and engineering applications. The Kelvin scale is absolute, meaning that it has a fixed zero point, which is defined as absolute zero (0 K).
The Boiling Point of Water in Kelvin
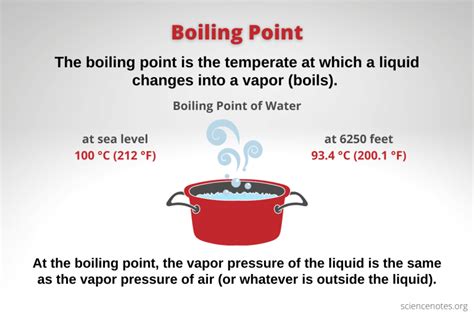
The boiling point of water at standard atmospheric pressure is 100 degrees Celsius (°C) or 212 degrees Fahrenheit (°F). However, when measured in Kelvin, the boiling point of water is 373.15 K. This value is obtained by adding 273.15 to the Celsius temperature, which is the conversion factor between Celsius and Kelvin.
Factors Affecting the Boiling Point of Water
Several factors can affect the boiling point of water, including:
-
Atmospheric Pressure
Atmospheric pressure has a significant impact on the boiling point of water. As pressure increases, the boiling point of water also increases. Conversely, as pressure decreases, the boiling point decreases. This is why water boils at a lower temperature at higher elevations, where atmospheric pressure is lower.

-
Impurities and Additives
The presence of impurities or additives can affect the boiling point of water. For example, adding salt or sugar to water can increase its boiling point, while the presence of air bubbles or other gases can decrease it.
-
Surface Tension
Surface tension is another factor that can affect the boiling point of water. Surface tension is the property of a liquid that causes it to behave as if it has an "elastic skin" at its surface. This skin can affect the rate of evaporation and, consequently, the boiling point of water.

Practical Applications of the Boiling Point of Water
The boiling point of water has numerous practical applications in various fields, including:
-
Cooking and Food Preparation
The boiling point of water is crucial in cooking and food preparation. It is used to cook pasta, rice, and other grains, as well as to sterilize equipment and utensils.
-
Steam Engines and Power Plants
The boiling point of water is used to generate steam in steam engines and power plants. Steam is used to drive turbines, which generate electricity.
-
Industrial Processes
The boiling point of water is used in various industrial processes, such as textile manufacturing, paper production, and chemical synthesis.

Conclusion and Future Directions
In conclusion, the boiling point of water in Kelvin is 373.15 K, which is a critical property that has numerous practical applications in various fields. Understanding the factors that affect the boiling point of water is essential for optimizing industrial processes, cooking, and other applications. Future research directions may include investigating the effects of new additives or impurities on the boiling point of water, as well as developing more efficient methods for measuring and controlling temperature.

Gallery of Related Images






What is the boiling point of water in Kelvin?
+The boiling point of water in Kelvin is 373.15 K.
What factors affect the boiling point of water?
+Atmospheric pressure, impurities, and additives can affect the boiling point of water.
What are some practical applications of the boiling point of water?
+Cooking, steam engines, and industrial processes are some practical applications of the boiling point of water.