Strontium, a chemical element with the symbol Sr and atomic number 38, is a soft, silvery alkaline earth metal. Understanding the valence electrons of strontium is crucial in chemistry and physics, as it helps us predict its reactivity and properties. Here are 5 key points to discover strontium's valence electrons:
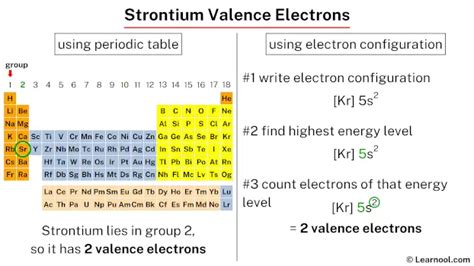
Strontium is an alkaline earth metal, which means it belongs to Group 2 of the periodic table. Elements in this group have two valence electrons, which are electrons in the outermost energy level of an atom. The valence electrons of strontium are responsible for its chemical properties and reactivity.
What are Valence Electrons?
Valence electrons are electrons in the outermost energy level of an atom. They are responsible for the chemical properties of an element, including its reactivity and ability to form compounds with other elements. Valence electrons are also known as outer electrons or electrons in the valence shell.

How Many Valence Electrons Does Strontium Have?
Strontium, like other alkaline earth metals, has two valence electrons. These electrons are located in the outermost energy level of the strontium atom and are responsible for its chemical properties. The electron configuration of strontium is [Kr] 5s2, which means it has two electrons in the 5s orbital.
Electron Configuration of Strontium
The electron configuration of strontium is [Kr] 5s2. This means that the strontium atom has a full outer energy level, with two electrons in the 5s orbital. The electron configuration of strontium is responsible for its chemical properties and reactivity.

What is the Significance of Valence Electrons in Strontium?
The valence electrons of strontium are significant because they determine its chemical properties and reactivity. The two valence electrons of strontium make it a reactive metal, which can easily lose electrons to form a positive ion. This reactivity makes strontium useful in various applications, including fireworks, magnets, and nuclear reactors.
Applications of Strontium
Strontium has several applications due to its unique properties. Some of the most common applications of strontium include:
- Fireworks: Strontium is used in fireworks to produce a red color.
- Magnets: Strontium is used in the production of magnets, which are used in a variety of applications, including electric motors and generators.
- Nuclear Reactors: Strontium is used in nuclear reactors as a fuel additive to improve the efficiency of the reactor.

Conclusion
In conclusion, strontium is an alkaline earth metal with two valence electrons. These electrons are responsible for its chemical properties and reactivity, making it a useful element in various applications. Understanding the valence electrons of strontium is crucial in chemistry and physics, as it helps us predict its reactivity and properties.





What is the atomic number of strontium?
+The atomic number of strontium is 38.
What is the electron configuration of strontium?
+The electron configuration of strontium is [Kr] 5s2.
What are the valence electrons of strontium?
+Strontium has two valence electrons.