The periodic table is a powerful tool for understanding the properties and behavior of elements. However, to gain a deeper understanding of an element's characteristics, we need to examine its atomic structure. One way to represent an element's atomic structure is through Lewis dot structures. In this article, we will explore the silicon Lewis dot structure, its significance, and how it relates to the element's properties.
What is a Lewis Dot Structure?
A Lewis dot structure, also known as an electron dot diagram, is a representation of an atom's valence electrons. It shows the number of electrons in an atom's outermost energy level, which determines the atom's reactivity and ability to form bonds with other atoms. The Lewis dot structure is a simple and effective way to visualize an atom's electronic configuration.
Silicon Lewis Dot Structure
Silicon, with an atomic number of 14, has 14 protons and 14 electrons in its atomic structure. The electron configuration of silicon is 1s² 2s² 2p⁶ 3s² 3p². The outermost energy level of silicon contains four electrons in the 3s and 3p orbitals.
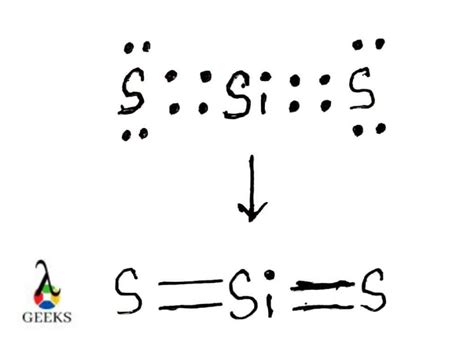
The Lewis dot structure of silicon is represented as follows:
In this representation, the silicon atom is surrounded by four dots, indicating the presence of four valence electrons. These electrons are arranged in a tetrahedral shape, with two electrons in the 3s orbital and two electrons in the 3p orbital.
Significance of Silicon Lewis Dot Structure
The silicon Lewis dot structure is crucial in understanding the element's chemical properties and behavior. Silicon's four valence electrons make it a tetravalent element, capable of forming four bonds with other atoms. This is reflected in silicon's ability to form a wide range of compounds, including silicates, silanes, and silicones.
The tetrahedral arrangement of silicon's valence electrons also explains its crystal structure. Silicon forms a diamond-like crystal lattice, with each silicon atom bonded to four neighboring atoms in a tetrahedral arrangement. This crystal structure is responsible for silicon's hardness, brittleness, and semiconductor properties.
Silicon's Unique Properties
Silicon's Lewis dot structure is also responsible for its unique properties, such as its ability to form a wide range of compounds and its semiconductor behavior. Silicon's four valence electrons allow it to form covalent bonds with other atoms, resulting in a wide range of compounds with diverse properties.
Silicon's semiconductor properties are also a result of its Lewis dot structure. The arrangement of silicon's valence electrons creates a small energy gap between the valence and conduction bands. This energy gap allows silicon to conduct electricity under certain conditions, making it a crucial material in the production of electronic devices.
How to Draw Silicon Lewis Dot Structure
Drawing the silicon Lewis dot structure is a simple process that requires an understanding of the element's electron configuration. Here's a step-by-step guide to drawing silicon's Lewis dot structure:
- Determine the number of valence electrons in silicon. Silicon has 14 electrons, with 4 electrons in its outermost energy level.
- Draw the symbol for silicon (Si).
- Arrange the four valence electrons in a tetrahedral shape around the silicon symbol.
- Ensure that each electron is represented by a single dot.
- Verify that the Lewis dot structure accurately represents the silicon atom's electronic configuration.
Conclusion
The silicon Lewis dot structure is a powerful tool for understanding the element's properties and behavior. By examining the arrangement of silicon's valence electrons, we can gain insights into its chemical reactivity, crystal structure, and semiconductor properties. The Lewis dot structure is a simple and effective way to visualize an atom's electronic configuration, making it an essential tool for chemists and materials scientists.
Benefits of Understanding Silicon Lewis Dot Structure
Understanding the silicon Lewis dot structure has numerous benefits in various fields, including:
- Materials Science: Understanding silicon's Lewis dot structure is crucial in developing new materials with unique properties.
- Electronics: Silicon's Lewis dot structure is essential in understanding its semiconductor properties and behavior.
- Chemistry: The Lewis dot structure is a fundamental tool in understanding chemical bonding and reactivity.
Common Applications of Silicon
Silicon is a versatile element with numerous applications in various fields, including:
- Electronics: Silicon is used in the production of microchips, solar panels, and other electronic devices.
- Construction: Silicon is used in the production of concrete, cement, and other construction materials.
- Medical: Silicon is used in medical implants, such as joint replacements and surgical instruments.
Gallery of Silicon-Related Images
FAQs
What is the Lewis dot structure of silicon?
+The Lewis dot structure of silicon is represented by four dots arranged in a tetrahedral shape around the silicon symbol.
Why is silicon's Lewis dot structure important?
+Silicon's Lewis dot structure is crucial in understanding its chemical properties and behavior, including its semiconductor properties and crystal structure.
What are some common applications of silicon?
+Silicon is used in various fields, including electronics, construction, and medicine. Some common applications include microchips, solar panels, and medical implants.
Share your thoughts and questions about silicon Lewis dot structure in the comments below!