The periodic table is a fundamental tool in chemistry, and understanding its various components is crucial for any aspiring chemist. One aspect of the periodic table that can be particularly fascinating is the concept of energy levels. In this article, we'll delve into the world of Period 3 energy levels, exploring their significance, characteristics, and practical applications.
Introduction to Energy Levels
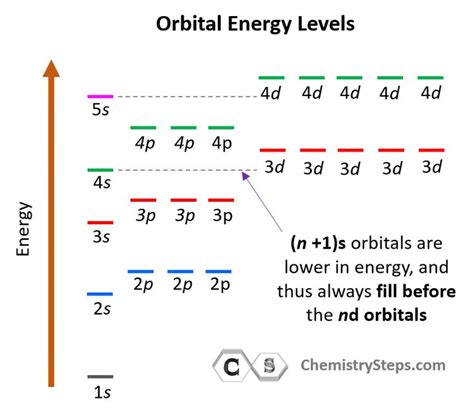
Before diving into Period 3, it's essential to understand the basics of energy levels. In chemistry, energy levels refer to the regions around an atom's nucleus where electrons are found. These levels are quantized, meaning they can only occupy specific energies. The energy levels are organized into shells, subshells, and orbitals, which determine the arrangement of electrons within an atom.
What is Period 3?
Period 3 is the third row of the periodic table, consisting of elements from sodium (Na) to argon (Ar). This period is particularly interesting because it marks a transition from the smaller, more compact atoms of Period 2 to the larger, more complex atoms of Period 3.
Characteristics of Period 3 Energy Levels
The energy levels in Period 3 exhibit several distinct characteristics:
- Shell and Subshell Structure: Period 3 elements have a 3s, 3p, and 3d subshell structure. The 3s subshell is filled first, followed by the 3p subshell, and finally the 3d subshell.
- Electron Configuration: The electron configuration of Period 3 elements is more complex than those in Period 2, with a greater number of electrons and a more intricate arrangement of subshells.
- Shielding Effect: The shielding effect, which occurs when inner electrons shield outer electrons from the nucleus, becomes more pronounced in Period 3. This results in a decrease in the effective nuclear charge, making it easier for electrons to be removed.
Significance of Period 3 Energy Levels
Understanding the energy levels in Period 3 is crucial for several reasons:
- Predicting Chemical Behavior: Knowing the energy levels of an element helps predict its chemical behavior, including its reactivity and the types of compounds it can form.
- Electron Configuration and Bonding: The energy levels of Period 3 elements determine their electron configuration, which in turn affects their ability to form bonds with other elements.
- Applications in Materials Science: Period 3 elements are used in a wide range of applications, from electronics to catalysis. Understanding their energy levels is essential for designing new materials with specific properties.

Practical Applications of Period 3 Energy Levels
The energy levels of Period 3 elements have numerous practical applications:
- Catalysis: Elements like aluminum (Al) and silicon (Si) are used as catalysts in various industrial processes, including the production of polyethylene and polypropylene.
- Electronics: The unique energy levels of Period 3 elements make them suitable for use in electronic devices, such as semiconductors and solar cells.
- Batteries: The high reactivity of elements like magnesium (Mg) and aluminum (Al) makes them useful in battery applications, including magnesium-ion and aluminum-ion batteries.
Conclusion
In conclusion, the energy levels of Period 3 elements are a fundamental aspect of chemistry, with significant implications for predicting chemical behavior, understanding electron configuration and bonding, and designing new materials. By grasping the characteristics and significance of Period 3 energy levels, chemists can unlock new possibilities in various fields, from materials science to electronics.
Gallery of Period 3 Elements





FAQ Section
What is the significance of Period 3 energy levels in chemistry?
+Period 3 energy levels are significant because they determine the chemical behavior of elements, including their reactivity and ability to form bonds with other elements.
How do the energy levels of Period 3 elements affect their electron configuration?
+The energy levels of Period 3 elements determine their electron configuration, which in turn affects their ability to form bonds with other elements.
What are some practical applications of Period 3 energy levels?
+Period 3 energy levels have numerous practical applications, including catalysis, electronics, and battery technology.
We hope this article has provided a comprehensive understanding of Period 3 energy levels in chemistry. Share your thoughts and questions in the comments below!