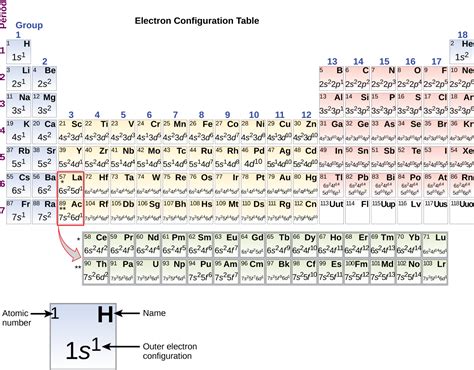
The periodic table is a powerful tool for understanding the properties and behavior of elements. One of the key concepts in chemistry is the valence electron, which plays a crucial role in determining the chemical properties of an element. In this article, we will delve into the world of valence electrons in Period 2 elements, exploring their importance, how they are configured, and what this means for the elements' chemical behavior.
What are Valence Electrons?
Valence electrons are the electrons in an atom that participate in chemical bonding. They are the outermost electrons in an atom and are responsible for the chemical properties of an element. Valence electrons are found in the outermost energy level of an atom, which is also known as the valence shell.

In Period 2 elements, the valence electrons are found in the second energy level. This energy level can hold a maximum of eight electrons, which is the reason why Period 2 elements have a range of chemical properties.
How are Valence Electrons Configured in Period 2 Elements?
The configuration of valence electrons in Period 2 elements follows the Aufbau principle and the Pauli Exclusion Principle. The Aufbau principle states that electrons occupy the lowest available energy levels, while the Pauli Exclusion Principle states that no two electrons can have the same set of quantum numbers.
In Period 2 elements, the valence electrons are configured in the 2s and 2p orbitals. The 2s orbital is spherical in shape and can hold a maximum of two electrons. The 2p orbitals are dumbbell-shaped and can hold a maximum of six electrons.

The electron configuration of Period 2 elements can be written as:
1s² 2s² 2p¹ (for boron) 1s² 2s² 2p² (for carbon) 1s² 2s² 2p³ (for nitrogen) 1s² 2s² 2p⁴ (for oxygen) 1s² 2s² 2p⁵ (for fluorine) 1s² 2s² 2p⁶ (for neon)
What is the Significance of Valence Electrons in Period 2 Elements?
The valence electrons in Period 2 elements play a crucial role in determining their chemical properties. The number of valence electrons an element has determines its reactivity, electronegativity, and ability to form chemical bonds.
Elements with a full outer energy level (such as neon) are unreactive, while elements with a partially filled outer energy level (such as carbon) are highly reactive. Elements with a high number of valence electrons (such as oxygen and fluorine) are highly electronegative and tend to form covalent bonds with other elements.

The valence electrons in Period 2 elements also determine their ability to form ions. Elements with a low number of valence electrons (such as boron) tend to lose electrons to form cations, while elements with a high number of valence electrons (such as oxygen and fluorine) tend to gain electrons to form anions.
Examples of Valence Electrons in Period 2 Elements
Here are some examples of how valence electrons affect the chemical properties of Period 2 elements:
- Carbon has four valence electrons, which makes it highly reactive and able to form a wide range of compounds.
- Oxygen has six valence electrons, which makes it highly electronegative and able to form covalent bonds with other elements.
- Fluorine has seven valence electrons, which makes it highly reactive and able to form covalent bonds with other elements.
- Neon has eight valence electrons, which makes it unreactive and unable to form chemical bonds with other elements.

Gallery of Period 2 Elements:






FAQ Section:
What are valence electrons?
+Valence electrons are the electrons in an atom that participate in chemical bonding. They are the outermost electrons in an atom and are responsible for the chemical properties of an element.
How are valence electrons configured in Period 2 elements?
+The valence electrons in Period 2 elements are configured in the 2s and 2p orbitals. The 2s orbital is spherical in shape and can hold a maximum of two electrons. The 2p orbitals are dumbbell-shaped and can hold a maximum of six electrons.
What is the significance of valence electrons in Period 2 elements?
+The valence electrons in Period 2 elements play a crucial role in determining their chemical properties. The number of valence electrons an element has determines its reactivity, electronegativity, and ability to form chemical bonds.
In conclusion, the valence electrons in Period 2 elements are a crucial aspect of their chemical properties. Understanding how these electrons are configured and how they affect the chemical behavior of elements can help us better understand the periodic table and the properties of elements. We hope this article has provided you with a comprehensive understanding of valence electrons in Period 2 elements. If you have any further questions or would like to learn more about this topic, please don't hesitate to ask.