The world of chemistry can be fascinating, yet complex, with various types of bonds that hold atoms together. One common compound that sparks curiosity is ammonia, denoted by the chemical formula NH3. When considering the nature of its bonding, a fundamental question arises: is NH3 covalent or ionic? To answer this, we need to delve into the basic principles of chemical bonding and explore the characteristics of covalent and ionic bonds.
Chemical Bonding: A Brief Overview
Chemical bonding is the process by which atoms share or exchange electrons to form a chemical compound. There are primarily three types of chemical bonds: covalent, ionic, and metallic. Each type is distinguished by the way atoms interact with each other.
Covalent Bonds: Sharing Electrons
Covalent bonds are formed when two or more atoms share one or more pairs of electrons to achieve a stable electronic configuration. This type of bonding typically occurs between non-metal atoms, which have a similar electronegativity. In a covalent bond, the electrons are shared equally or unequally, depending on the difference in electronegativity between the atoms.

Ionic Bonds: Transferring Electrons
Ionic bonds, on the other hand, are formed when one or more electrons are transferred from a metal atom to a non-metal atom. This transfer of electrons results in the formation of ions with opposite charges, which are then attracted to each other. Ionic bonds typically occur between metal and non-metal atoms, which have a significant difference in electronegativity.

NH3: A Covalent Compound
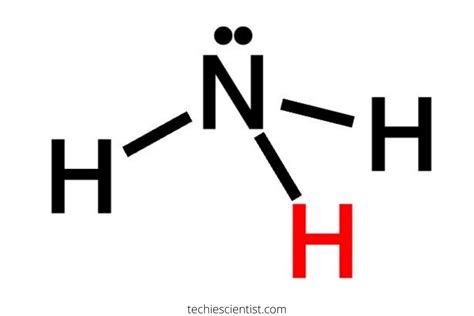
Now, let's examine the bonding in ammonia (NH3). Ammonia consists of one nitrogen atom bonded to three hydrogen atoms. The nitrogen atom has five valence electrons, while each hydrogen atom has one valence electron. To achieve a stable electronic configuration, the nitrogen atom shares its three unpaired electrons with the three hydrogen atoms, forming three covalent bonds.
The nitrogen-hydrogen bonds in ammonia are polar covalent bonds, meaning that the electrons are not shared equally between the atoms. The nitrogen atom has a higher electronegativity than the hydrogen atoms, resulting in a slight positive charge on the hydrogen atoms and a slight negative charge on the nitrogen atom.

Conclusion
In conclusion, NH3 is a covalent compound, where the nitrogen atom shares its electrons with the three hydrogen atoms to form three polar covalent bonds. The unequal sharing of electrons results in a partial positive charge on the hydrogen atoms and a partial negative charge on the nitrogen atom. Understanding the type of bonding in ammonia is essential for recognizing its chemical properties and behavior.
We hope this article has helped you grasp the concept of covalent and ionic bonds and how they apply to ammonia. Share your thoughts and questions in the comments section below!
Gallery of Covalent and Ionic Bonds






What is the difference between covalent and ionic bonds?
+Covalent bonds are formed when atoms share electrons, while ionic bonds are formed when electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges.
What type of bond is present in ammonia (NH3)?
+Ammonia (NH3) contains polar covalent bonds between the nitrogen and hydrogen atoms.
What is the significance of electronegativity in chemical bonding?
+Electronegativity plays a crucial role in determining the type of bond formed between atoms. A large difference in electronegativity typically results in ionic bonding, while a small difference results in covalent bonding.