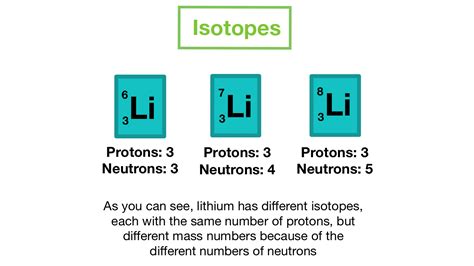
Sodium, a chemical element with the symbol Na, is a highly reactive metal that is essential for various industrial, biological, and household applications. Its isotopes, which are atoms of the same element with different numbers of neutrons, play a crucial role in understanding the properties and behavior of sodium. Among the 19 known isotopes of sodium, five naturally occurring isotopes are worth highlighting. These isotopes are crucial in various fields, including medicine, agriculture, and environmental science.

Natural Sodium Isotopes: Overview
Natural sodium isotopes are the isotopes that occur naturally on Earth. They are formed through various processes, including radioactive decay, nuclear reactions, and cosmogenic processes. These isotopes have different neutron numbers, which affect their stability, half-life, and reactivity.
Importance of Natural Sodium Isotopes
Natural sodium isotopes are essential in various fields, including:
- Medicine: Sodium isotopes are used in medical imaging and diagnostics.
- Agriculture: Sodium isotopes are used to study plant nutrition and soil science.
- Environmental science: Sodium isotopes are used to study environmental processes, such as weathering and erosion.
Five Natural Sodium Isotopes
1. Sodium-23 (Na-23)
Sodium-23 is the only stable isotope of sodium, making up 100% of natural sodium. It has 11 protons and 12 neutrons in its atomic nucleus.

2. Sodium-24 (Na-24)
Sodium-24 is a radioactive isotope with a half-life of approximately 15 hours. It is formed through the neutron activation of sodium-23.

3. Sodium-22 (Na-22)
Sodium-22 is a radioactive isotope with a half-life of approximately 2.6 years. It is formed through the cosmic ray spallation of argon.

4. Sodium-20 (Na-20)
Sodium-20 is a radioactive isotope with a half-life of approximately 446 milliseconds. It is formed through the beta decay of neon-20.

5. Sodium-26 (Na-26)
Sodium-26 is a radioactive isotope with a half-life of approximately 1.07 seconds. It is formed through the beta decay of magnesium-26.

Applications of Natural Sodium Isotopes
Natural sodium isotopes have various applications in medicine, agriculture, and environmental science. For example, sodium-24 is used in medical imaging, while sodium-22 is used in agricultural research.
Medical Applications
Sodium isotopes are used in medical imaging and diagnostics. For example, sodium-24 is used in positron emission tomography (PET) scans to study brain function.
Agricultural Applications
Sodium isotopes are used in agricultural research to study plant nutrition and soil science. For example, sodium-22 is used to study the uptake of sodium by plants.
Environmental Applications
Sodium isotopes are used in environmental science to study environmental processes, such as weathering and erosion. For example, sodium-23 is used to study the weathering of rocks.





What are sodium isotopes?
+Sodium isotopes are atoms of the same element with different numbers of neutrons.
What are the five natural sodium isotopes?
+The five natural sodium isotopes are sodium-23, sodium-24, sodium-22, sodium-20, and sodium-26.
What are the applications of natural sodium isotopes?
+Natural sodium isotopes have various applications in medicine, agriculture, and environmental science.
We hope this article has provided you with a comprehensive understanding of the five natural sodium isotopes. If you have any further questions or would like to know more about sodium isotopes, please don't hesitate to comment below.