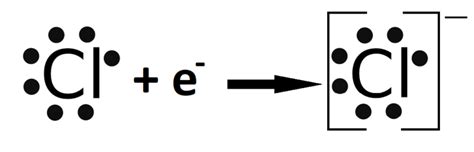The N-Anion Lewis Structure is a fundamental concept in chemistry, particularly in the field of organic chemistry. It is a crucial tool for understanding the properties and behavior of molecules, and is used extensively in various fields such as pharmaceuticals, materials science, and biochemistry. In this article, we will delve into the world of N-Anion Lewis Structure, exploring its definition, importance, and applications.
What is N-Anion Lewis Structure?
The N-Anion Lewis Structure, also known as the nitride ion, is a type of Lewis structure that represents the electron configuration of a nitrogen atom with an extra electron. It is a negatively charged ion, denoted by the symbol N3-. The Lewis structure of N-Anion consists of a nitrogen atom surrounded by three pairs of electrons, with a single electron occupying the outermost energy level.

Importance of N-Anion Lewis Structure
The N-Anion Lewis Structure plays a vital role in understanding the properties and behavior of molecules. It is used to predict the reactivity of molecules, identify the most stable configuration, and determine the polarity of bonds. The N-Anion Lewis Structure is also essential in understanding the mechanisms of chemical reactions, particularly those involving nitrogen-containing compounds.
How to Draw N-Anion Lewis Structure
Drawing the N-Anion Lewis Structure is a straightforward process that involves a few simple steps. Here's a step-by-step guide:
- Start by drawing the nitrogen atom, represented by the symbol N.
- Add three pairs of electrons to the nitrogen atom, representing the three pairs of electrons in the outermost energy level.
- Add a single electron to the outermost energy level, representing the extra electron that gives the N-Anion its negative charge.
- Arrange the electrons in a way that minimizes electron-electron repulsion, resulting in a trigonal pyramidal shape.

Applications of N-Anion Lewis Structure
The N-Anion Lewis Structure has numerous applications in various fields, including:
- Pharmaceuticals: The N-Anion Lewis Structure is used to design and develop new drugs, particularly those containing nitrogen-based functional groups.
- Materials Science: The N-Anion Lewis Structure is used to understand the properties of materials, such as conductivity and optical properties.
- Biochemistry: The N-Anion Lewis Structure is used to understand the mechanisms of biological reactions, particularly those involving nitrogen-containing compounds.

Conclusion and Future Directions
In conclusion, the N-Anion Lewis Structure is a fundamental concept in chemistry that plays a vital role in understanding the properties and behavior of molecules. Its importance extends beyond the field of chemistry, with applications in pharmaceuticals, materials science, and biochemistry. As research continues to advance, the N-Anion Lewis Structure will remain a crucial tool for understanding the intricacies of molecular behavior.






What is the N-Anion Lewis Structure?
+The N-Anion Lewis Structure is a type of Lewis structure that represents the electron configuration of a nitrogen atom with an extra electron.
Why is the N-Anion Lewis Structure important?
+The N-Anion Lewis Structure plays a vital role in understanding the properties and behavior of molecules, particularly those containing nitrogen-based functional groups.
What are some applications of the N-Anion Lewis Structure?
+The N-Anion Lewis Structure has numerous applications in various fields, including pharmaceuticals, materials science, and biochemistry.