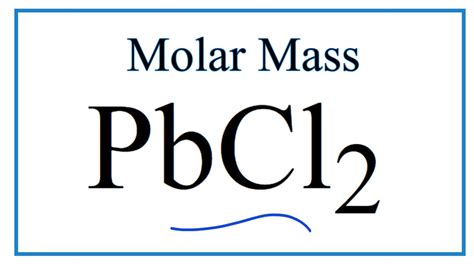In the world of chemistry, understanding the properties and characteristics of various compounds is crucial for both researchers and students. One such compound that has garnered significant attention in recent years is PbCl2, also known as lead(II) chloride. In this article, we will delve into the world of PbCl2, focusing specifically on its molar mass and the importance of this property in various chemical applications.
What is PbCl2?
Before we dive into the molar mass of PbCl2, it's essential to understand what this compound is and its chemical structure. PbCl2 is a white crystalline solid with a chemical formula of PbCl2, indicating that one lead atom is bonded to two chlorine atoms. This compound is highly insoluble in water and has a wide range of applications in various industries, including the manufacturing of batteries, ammunition, and glass.
Importance of Molar Mass
In chemistry, the molar mass of a compound is a critical property that determines its behavior and reactivity. The molar mass is the sum of the atomic masses of all the atoms present in a molecule. It is expressed in units of grams per mole (g/mol) and is a fundamental concept in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products.
PbCl2 Molar Mass
So, what is the molar mass of PbCl2? To calculate the molar mass of PbCl2, we need to sum the atomic masses of lead (Pb) and chlorine (Cl). The atomic mass of lead is 207.2 g/mol, while the atomic mass of chlorine is 35.45 g/mol.
PbCl2 molar mass = (1 x atomic mass of Pb) + (2 x atomic mass of Cl) = (1 x 207.2 g/mol) + (2 x 35.45 g/mol) = 207.2 g/mol + 70.9 g/mol = 278.1 g/mol
Therefore, the molar mass of PbCl2 is approximately 278.1 g/mol.

Applications of PbCl2 Molar Mass
The molar mass of PbCl2 has significant implications in various chemical applications. Here are a few examples:
- Battery manufacturing: PbCl2 is used as a precursor material in the production of lead-acid batteries. Knowing the molar mass of PbCl2 is crucial in determining the correct stoichiometry of the reaction.
- Ammunition production: PbCl2 is used as a stabilizer in the production of lead-based ammunition. The molar mass of PbCl2 is essential in determining the correct amount of stabilizer required.
- Glass manufacturing: PbCl2 is used as a flux in the production of lead glass. The molar mass of PbCl2 is critical in determining the correct amount of flux required to achieve the desired properties.
Why is PbCl2 Molar Mass Important?
The molar mass of PbCl2 is important for several reasons:
- Accurate calculations: Knowing the molar mass of PbCl2 allows chemists to perform accurate calculations in various chemical reactions and applications.
- Stoichiometry: The molar mass of PbCl2 is essential in determining the correct stoichiometry of reactions, which is critical in achieving the desired outcome.
- Material properties: The molar mass of PbCl2 can affect the material properties of the compound, such as its density and solubility.
How to Calculate PbCl2 Molar Mass
Calculating the molar mass of PbCl2 is a straightforward process that involves summing the atomic masses of lead and chlorine. Here's a step-by-step guide:
- Determine the atomic mass of lead (Pb) and chlorine (Cl).
- Sum the atomic masses of lead and chlorine to get the molar mass of PbCl2.
- Express the molar mass in units of grams per mole (g/mol).

Common Mistakes in PbCl2 Molar Mass Calculation
When calculating the molar mass of PbCl2, it's essential to avoid common mistakes that can lead to inaccurate results. Here are a few common mistakes to watch out for:
- Incorrect atomic masses: Ensure that you use the correct atomic masses for lead and chlorine.
- Incorrect stoichiometry: Ensure that you use the correct stoichiometry of the reaction to determine the correct amount of PbCl2 required.
Conclusion
In conclusion, the molar mass of PbCl2 is a critical property that has significant implications in various chemical applications. By understanding the molar mass of PbCl2, chemists and researchers can perform accurate calculations, determine the correct stoichiometry of reactions, and achieve the desired material properties. We hope that this article has provided you with a comprehensive understanding of PbCl2 molar mass and its importance in chemistry.
Gallery of PbCl2-related images





FAQs
What is the molar mass of PbCl2?
+The molar mass of PbCl2 is approximately 278.1 g/mol.
What is the atomic mass of lead?
+The atomic mass of lead is 207.2 g/mol.
What is the atomic mass of chlorine?
+The atomic mass of chlorine is 35.45 g/mol.