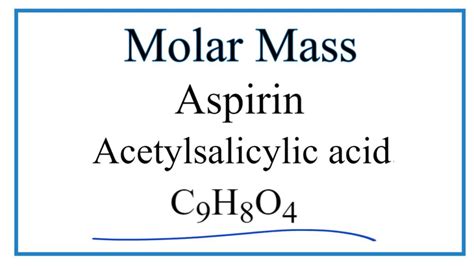
The Molar Mass of Acetylsalicylic Acid: Understanding the Chemistry Behind Aspirin
Acetylsalicylic acid, commonly known as aspirin, is a widely used medication for relieving pain, reducing inflammation, and preventing blood clots. But have you ever wondered about the chemistry behind this ubiquitous drug? In this article, we'll delve into the world of organic chemistry and explore the molar mass of acetylsalicylic acid.
What is Acetylsalicylic Acid?
Acetylsalicylic acid is a derivative of salicylic acid, which is found naturally in the bark of the willow tree. The addition of an acetyl group to salicylic acid creates a compound with unique properties that make it useful for medicinal purposes. Acetylsalicylic acid is a white, crystalline powder with a characteristic odor and a bitter taste.
The Chemical Structure of Acetylsalicylic Acid
The chemical formula for acetylsalicylic acid is C9H8O4. Its molecular structure consists of a benzene ring with a hydroxyl group, a carboxyl group, and an acetyl group attached to it. The arrangement of these functional groups gives acetylsalicylic acid its distinctive properties and reactivity.
Calculating the Molar Mass of Acetylsalicylic Acid
The molar mass of a compound is the sum of the atomic masses of its constituent elements. To calculate the molar mass of acetylsalicylic acid, we need to know the atomic masses of carbon, hydrogen, and oxygen.
- Atomic mass of carbon (C): 12.01 g/mol
- Atomic mass of hydrogen (H): 1.008 g/mol
- Atomic mass of oxygen (O): 16.00 g/mol
Using the chemical formula C9H8O4, we can calculate the molar mass of acetylsalicylic acid as follows:
Molar mass = (9 x 12.01) + (8 x 1.008) + (4 x 16.00) Molar mass = 108.09 + 8.064 + 64.00 Molar mass = 180.154 g/mol
Rounding the Molar Mass
The calculated molar mass of acetylsalicylic acid is 180.154 g/mol. However, it's common to round this value to the nearest whole number or to two decimal places. In this case, we can round the molar mass to 180.15 g/mol.
Applications of Acetylsalicylic Acid
Acetylsalicylic acid has a wide range of applications in medicine and industry. Some of its most common uses include:
- Pain relief: Aspirin is widely used to relieve headaches, muscle aches, and joint pain.
- Anti-inflammatory: Aspirin has anti-inflammatory properties, making it useful for reducing swelling and inflammation.
- Antipyretic: Aspirin can help lower fever and reduce the risk of complications associated with high temperatures.
- Anticoagulant: Aspirin can help prevent blood clots from forming, making it useful for preventing heart attacks and strokes.
Printable Resources
For those interested in learning more about acetylsalicylic acid, we've provided some printable resources below:


Gallery of Acetylsalicylic Acid





FAQs
What is the chemical formula for acetylsalicylic acid?
+The chemical formula for acetylsalicylic acid is C9H8O4.
What is the molar mass of acetylsalicylic acid?
+The molar mass of acetylsalicylic acid is 180.15 g/mol.
What are some common uses of acetylsalicylic acid?
+Acetylsalicylic acid is commonly used for pain relief, reducing inflammation, and preventing blood clots.
We hope this article has provided you with a deeper understanding of the chemistry behind acetylsalicylic acid. Whether you're a student, a researcher, or simply someone interested in learning more about the world around you, we encourage you to explore the many resources available on this topic.