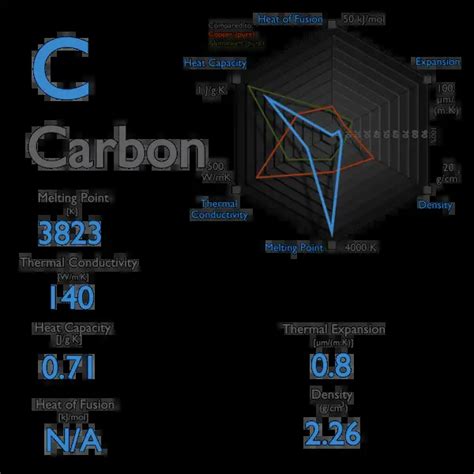
Glassy carbon, a non-graphitizing form of carbon, has been widely used in various applications, including electrochemistry, catalysis, and biomedical research. One of the key properties of glassy carbon is its melting point, which is a crucial factor in determining its suitability for specific applications. In this article, we will delve into the concept of melting point, its significance, and the specific melting point of glassy carbon.
What is Melting Point?
The melting point is the temperature at which a solid changes its state to become a liquid. At this temperature, the solid's crystal structure breaks down, and the molecules begin to move freely, resulting in a liquid state. The melting point is a fundamental property of materials and is used to characterize their thermal behavior.

Why is Melting Point Important?
The melting point is a critical property in materials science, as it affects the material's performance, durability, and suitability for specific applications. A material's melting point can influence its:
- Thermal stability: A high melting point indicates a material's ability to withstand high temperatures without deforming or losing its shape.
- Chemical reactivity: The melting point can affect a material's reactivity, as some materials may undergo chemical reactions or decomposition at high temperatures.
- Mechanical properties: The melting point can impact a material's mechanical properties, such as strength, toughness, and hardness.
Melting Point of Glassy Carbon
Glassy carbon, a non-crystalline form of carbon, has a unique melting point. Unlike graphite, which has a well-defined melting point, glassy carbon's melting point is not precisely defined. However, it is generally accepted that glassy carbon's melting point lies in the range of 2500-3000°C (4500-5500°F).

Factors Affecting Glassy Carbon's Melting Point
Several factors can influence the melting point of glassy carbon, including:
- Purity: The presence of impurities can affect the melting point of glassy carbon.
- Density: Glassy carbon's density can impact its melting point, as denser materials tend to have higher melting points.
- Surface roughness: The surface roughness of glassy carbon can also influence its melting point, as rough surfaces can lead to a lower melting point.
Applications of Glassy Carbon
Glassy carbon's unique properties, including its melting point, make it an ideal material for various applications, such as:
- Electrochemistry: Glassy carbon is widely used as an electrode material in electrochemical applications due to its high surface area, conductivity, and corrosion resistance.
- Catalysis: Glassy carbon's high surface area and thermal stability make it an effective catalyst support material.
- Biomedical research: Glassy carbon's biocompatibility and corrosion resistance make it a suitable material for biomedical applications, such as implants and biosensors.

Conclusion
In conclusion, the melting point of glassy carbon is a critical property that affects its suitability for various applications. Understanding the factors that influence glassy carbon's melting point is essential for optimizing its performance and durability. As research continues to uncover the unique properties of glassy carbon, its applications are likely to expand, making it an increasingly important material in various fields.






What is the melting point of glassy carbon?
+The melting point of glassy carbon is generally accepted to be in the range of 2500-3000°C (4500-5500°F).
What factors affect the melting point of glassy carbon?
+The melting point of glassy carbon can be influenced by factors such as purity, density, and surface roughness.
What are some common applications of glassy carbon?
+Glassy carbon is widely used in electrochemistry, catalysis, and biomedical research due to its unique properties.