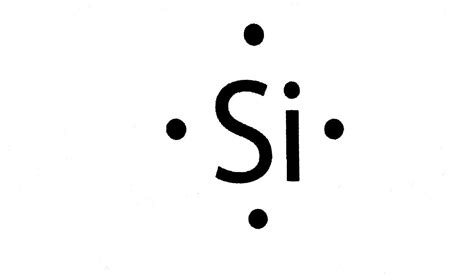Silicon dioxide, also known as silica, is one of the most common compounds found in nature. It is a major component of sand and quartz, and is also found in many types of rocks and minerals. Understanding the Lewis structure of silicon dioxide is essential for understanding its properties and behavior.
In this article, we will take a closer look at the Lewis structure of silicon dioxide, including its molecular formula, electron geometry, and bond angles. We will also discuss the importance of silicon dioxide in various industries and its role in everyday life.
What is Silicon Dioxide?
Silicon dioxide, also known as silica, is a compound made up of silicon and oxygen atoms. Its molecular formula is SiO2, indicating that one silicon atom is bonded to two oxygen atoms. Silicon dioxide is a naturally occurring compound that can be found in many types of rocks and minerals, including sand, quartz, and granite.
Lewis Structure of Silicon Dioxide
The Lewis structure of silicon dioxide is a diagram that shows the arrangement of electrons in the molecule. It is a simple and effective way to visualize the molecule and understand its properties.
To draw the Lewis structure of silicon dioxide, we start by writing the molecular formula, SiO2. We then draw a single bond between the silicon atom and each of the two oxygen atoms.
In the Lewis structure, the silicon atom is bonded to two oxygen atoms, each with a single bond. The silicon atom has four valence electrons, which are shared with the oxygen atoms to form the covalent bonds.
Electron Geometry and Bond Angles
The electron geometry of silicon dioxide is tetrahedral, meaning that the silicon atom is bonded to four oxygen atoms in a three-dimensional arrangement. However, the molecular geometry is bent or V-shaped, due to the lone pairs of electrons on the oxygen atoms.
The bond angles in silicon dioxide are approximately 109.5 degrees, which is the same as the bond angles in a tetrahedral molecule. However, the presence of lone pairs on the oxygen atoms causes the bond angles to be slightly less than the ideal tetrahedral angle.
Importance of Silicon Dioxide
Silicon dioxide is an important compound with many uses in various industries. Some of the most significant uses of silicon dioxide include:
- Construction: Silicon dioxide is a major component of concrete and cement, which are used to build roads, bridges, and buildings.
- Electronics: Silicon dioxide is used as a semiconductor material in the production of computer chips and other electronic devices.
- Glassmaking: Silicon dioxide is a major component of glass, which is used to make windows, bottles, and other glass products.
- Cosmetics: Silicon dioxide is used as an abrasive in toothpaste and other personal care products.
Role in Everyday Life
Silicon dioxide plays a significant role in our everyday lives, from the construction of buildings and roads to the production of electronic devices and glass products. It is also used in many personal care products, such as toothpaste and cosmetics.
In addition, silicon dioxide is an important component of many types of rocks and minerals, including sand and quartz. These rocks and minerals are used in a variety of applications, including construction, landscaping, and decorative stone.
Gallery of Silicon Dioxide
FAQs
What is the molecular formula of silicon dioxide?
+The molecular formula of silicon dioxide is SiO2.
What is the electron geometry of silicon dioxide?
+The electron geometry of silicon dioxide is tetrahedral.
What are some common uses of silicon dioxide?
+Silicon dioxide is used in construction, electronics, glassmaking, and cosmetics.
In conclusion, silicon dioxide is an important compound with many uses in various industries. Understanding its Lewis structure, electron geometry, and bond angles is essential for understanding its properties and behavior. We hope this article has provided a clear and concise explanation of the Lewis structure of silicon dioxide and its significance in everyday life.