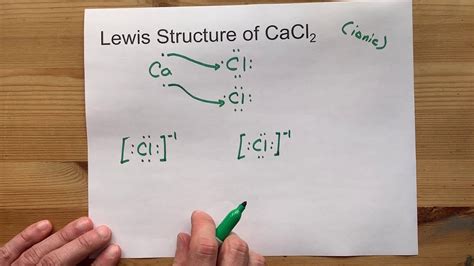Drawing Lewis structures can be a daunting task for many students, but with a few simple steps, you can master the process. In this article, we will focus on drawing the Lewis structure of CaCl2, also known as calcium chloride.
What is a Lewis Structure?
A Lewis structure is a diagram that represents the bonding between atoms in a molecule. It is a simplified representation of the molecular structure, using symbols and lines to show the arrangement of electrons. Lewis structures are essential in understanding the chemical properties and behavior of molecules.

Why Draw Lewis Structures?
Drawing Lewis structures helps you understand the molecular geometry, polarity, and reactivity of a molecule. It also provides a visual representation of the molecule's electron distribution, which is essential in understanding chemical reactions and bonding.
Benefits of Drawing Lewis Structures
- Understand molecular geometry and polarity
- Identify reactive sites and functional groups
- Predict chemical reactivity and properties
- Visualize electron distribution and bonding

How to Draw the Lewis Structure of CaCl2
Drawing the Lewis structure of CaCl2 involves a few simple steps:
- Determine the total number of valence electrons: Calcium (Ca) has 2 valence electrons, and each Chlorine (Cl) atom has 7 valence electrons. Therefore, the total number of valence electrons is 2 + 2(7) = 16.
- Draw the skeleton structure: Place the Calcium atom in the center, and the two Chlorine atoms on either side.
- Distribute the electrons: Place two electrons between the Calcium and each Chlorine atom to form a covalent bond.
- Complete the octet: Each Chlorine atom should have eight electrons in its valence shell. Add the remaining electrons to the Chlorine atoms to complete their octet.

Tips and Tricks
- Always start with the least electronegative atom (Calcium in this case).
- Use the octet rule to determine the number of electrons needed to complete the valence shell.
- Be careful not to exceed the octet rule, as this can lead to incorrect structures.

Conclusion
Drawing Lewis structures is an essential skill in chemistry, and with practice, you can master the process. Remember to follow the simple steps outlined in this article, and don't hesitate to ask for help if you need it. Happy drawing!





What is the purpose of drawing Lewis structures?
+Drawing Lewis structures helps you understand the molecular geometry, polarity, and reactivity of a molecule.
How many valence electrons does Calcium have?
+Calcium has 2 valence electrons.
What is the octet rule?
+The octet rule states that each atom should have eight electrons in its valence shell.