The concept of absolute zero has fascinated scientists and philosophers for centuries. It is a fundamental idea in physics that has far-reaching implications for our understanding of the universe. In this article, we will delve into the concept of absolute zero, its significance, and its applications.
What is Absolute Zero?
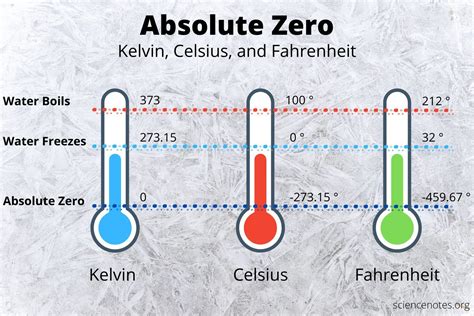
Absolute zero is the lowest possible temperature that can be achieved, defined as 0 Kelvin (K), -273.15 degrees Celsius (°C), or -459.67 degrees Fahrenheit (°F). It is the point at which all matter would theoretically have zero entropy, meaning that all molecular motion would cease. This concept was first proposed by the French physicist Guillaume Amontons in 1702 and later developed by Lord Kelvin in the 19th century.

The Significance of Absolute Zero
Absolute zero is a theoretical concept, and it is impossible to achieve in practice. However, the idea of absolute zero has far-reaching implications for our understanding of the universe. It provides a fundamental limit for the behavior of matter and energy, and it has led to significant advances in fields such as cryogenics, materials science, and quantum mechanics.
Approaching Absolute Zero
While it is impossible to achieve absolute zero, scientists have been able to get extremely close. The current record for the lowest temperature achieved is around 450 picokelvin (pK), which is just a few billionths of a degree above absolute zero. This was achieved using advanced cryogenic techniques, such as laser cooling and magnetic trapping.

Applications of Absolute Zero
The concept of absolute zero has led to significant advances in various fields, including:
- Cryogenics: The study of the behavior of materials at extremely low temperatures has led to the development of new materials and technologies, such as superconductors and superfluids.
- Materials Science: The understanding of the behavior of materials at absolute zero has led to the development of new materials with unique properties, such as nanomaterials and metamaterials.
- Quantum Mechanics: The concept of absolute zero has led to a deeper understanding of the behavior of matter at the quantum level, which has significant implications for our understanding of the universe.
Theoretical Implications of Absolute Zero
The concept of absolute zero has significant implications for our understanding of the universe. It provides a fundamental limit for the behavior of matter and energy, and it has led to significant advances in our understanding of the universe.

The Third Law of Thermodynamics
The concept of absolute zero is closely related to the third law of thermodynamics, which states that as the temperature of a system approaches absolute zero, its entropy approaches a minimum value. This law has significant implications for our understanding of the behavior of matter and energy at the quantum level.
Conclusion
In conclusion, the concept of absolute zero is a fundamental idea in physics that has far-reaching implications for our understanding of the universe. While it is impossible to achieve in practice, the idea of absolute zero has led to significant advances in fields such as cryogenics, materials science, and quantum mechanics. As scientists continue to explore the mysteries of the universe, the concept of absolute zero will remain a fundamental limit for the behavior of matter and energy.






What is absolute zero?
+Absolute zero is the lowest possible temperature that can be achieved, defined as 0 Kelvin (K), -273.15 degrees Celsius (°C), or -459.67 degrees Fahrenheit (°F).
Why is it impossible to achieve absolute zero?
+It is impossible to achieve absolute zero because it would require the removal of all entropy from a system, which is theoretically impossible.
What are the applications of absolute zero?
+The concept of absolute zero has led to significant advances in fields such as cryogenics, materials science, and quantum mechanics.