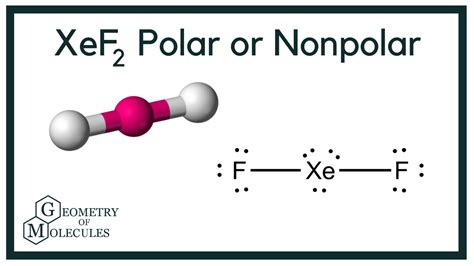
Xenon difluoride, or XeF2, is a chemical compound that has been the subject of much interest and debate among chemists and scientists. One of the key questions surrounding XeF2 is whether it is a polar or nonpolar molecule. In this article, we will delve into the world of molecular polarity and explore the characteristics of XeF2 to determine whether it is polar or nonpolar.
Understanding Molecular Polarity
Before we can determine whether XeF2 is polar or nonpolar, we need to understand what molecular polarity is. Molecular polarity refers to the distribution of electric charge within a molecule. In a polar molecule, the electric charge is not evenly distributed, resulting in a molecule with a slightly positive charge on one end and a slightly negative charge on the other. This creates a dipole moment, which is a measure of the molecule's polarity.
On the other hand, nonpolar molecules have an even distribution of electric charge, resulting in no net dipole moment. Nonpolar molecules are typically symmetrical, with the electric charge evenly distributed around the molecule.

The Structure of XeF2
To determine whether XeF2 is polar or nonpolar, we need to examine its molecular structure. XeF2 is a linear molecule, consisting of a central xenon atom bonded to two fluorine atoms. The xenon atom is a noble gas, which means it is unreactive and has a full outer energy level. The fluorine atoms, on the other hand, are highly reactive and have a strong tendency to attract electrons.
The bond between the xenon and fluorine atoms is a covalent bond, meaning that the electrons are shared between the atoms. However, due to the difference in electronegativity between the xenon and fluorine atoms, the electrons are not shared equally. The fluorine atoms have a higher electronegativity than the xenon atom, which means they have a stronger tendency to attract electrons.
Electronegativity and Polarity
The difference in electronegativity between the xenon and fluorine atoms is the key to understanding the polarity of XeF2. The fluorine atoms are more electronegative than the xenon atom, which means they have a stronger tendency to attract electrons. This results in a slightly negative charge on the fluorine atoms and a slightly positive charge on the xenon atom.
As a result, XeF2 has a net dipole moment, which means it is a polar molecule. The dipole moment is relatively small, but it is still present.
Factors Affecting Polarity
There are several factors that can affect the polarity of a molecule, including electronegativity, molecular shape, and bond length. In the case of XeF2, the difference in electronegativity between the xenon and fluorine atoms is the primary factor affecting its polarity.
However, the molecular shape of XeF2 also plays a role in its polarity. The linear shape of the molecule means that the dipole moment is not cancelled out by symmetry, as it would be in a molecule with a symmetrical shape.

Practical Applications of XeF2
XeF2 has several practical applications, including its use as a fluorinating agent in organic chemistry. Its polar nature makes it useful for a variety of chemical reactions, including the fluorination of alkenes and alkynes.
In addition, XeF2 has been used as a laser medium, due to its ability to emit light in the ultraviolet region of the spectrum.
Conclusion
In conclusion, XeF2 is a polar molecule due to the difference in electronegativity between the xenon and fluorine atoms. Its linear shape and bond length also play a role in its polarity, but the difference in electronegativity is the primary factor.
Understanding the polarity of XeF2 is important for a variety of practical applications, including its use as a fluorinating agent and laser medium. By understanding the characteristics of XeF2, we can better appreciate its uses and limitations.






We hope this article has helped you understand the polarity of XeF2. If you have any questions or comments, please feel free to ask.
What is molecular polarity?
+Molecular polarity refers to the distribution of electric charge within a molecule. In a polar molecule, the electric charge is not evenly distributed, resulting in a molecule with a slightly positive charge on one end and a slightly negative charge on the other.
What is the difference between polar and nonpolar molecules?
+Polar molecules have an uneven distribution of electric charge, resulting in a molecule with a slightly positive charge on one end and a slightly negative charge on the other. Nonpolar molecules have an even distribution of electric charge, resulting in no net dipole moment.
What is the structure of XeF2?
+XeF2 is a linear molecule, consisting of a central xenon atom bonded to two fluorine atoms.