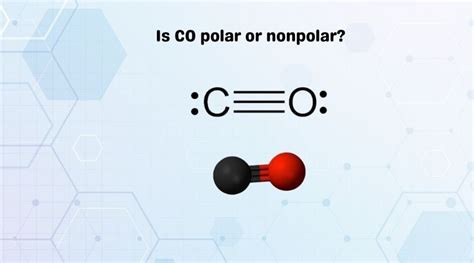
Carbon monoxide, or CO, is a molecule composed of one carbon atom and one oxygen atom. While it may seem simple, the question of whether CO is a polar molecule is a great opportunity to dive into the world of chemistry and understand the underlying principles.
In this article, we'll explore what makes a molecule polar, the properties of carbon monoxide, and provide a clear explanation of why CO is or isn't a polar molecule.
What is a Polar Molecule?
A polar molecule is a molecule that has a net dipole moment, meaning it has a slightly positive charge on one end and a slightly negative charge on the other end. This occurs when the electrons in the molecule are not shared equally between the atoms, resulting in a molecule with a permanent electric dipole moment.
Polar molecules are typically found in molecules with a difference in electronegativity between the atoms, meaning one atom has a stronger pull on the shared electrons than the other atom. This difference in electronegativity creates a slight imbalance of charge, resulting in a polar molecule.

Properties of Carbon Monoxide
Carbon monoxide is a colorless, odorless, and highly toxic gas. It is a simple molecule composed of one carbon atom and one oxygen atom, with a triple bond between the two atoms.
The electronegativity of carbon is 2.5, while the electronegativity of oxygen is 3.4. This means that oxygen has a slightly stronger pull on the shared electrons in the CO molecule.
Electronegativity Difference
The difference in electronegativity between carbon and oxygen is 0.9. While this may seem significant, it's essential to consider the overall molecule and its structure.
In CO, the triple bond between the carbon and oxygen atoms is polarized, meaning the electrons are not shared equally between the atoms. However, the molecule as a whole is still relatively non-polar due to the symmetrical distribution of electrons.

Is CO a Polar Molecule?
Based on the properties of carbon monoxide, it's clear that the molecule has a slight imbalance of charge due to the difference in electronegativity between the carbon and oxygen atoms.
However, the CO molecule is still considered a non-polar molecule. While the triple bond is polarized, the molecule as a whole has a symmetrical distribution of electrons, resulting in no net dipole moment.
The slight imbalance of charge in the CO molecule is not enough to classify it as a polar molecule. Instead, CO is considered a non-polar molecule with a slight dipole moment.

Conclusion
In conclusion, while the CO molecule has a slight imbalance of charge due to the difference in electronegativity between the carbon and oxygen atoms, it is still considered a non-polar molecule. The symmetrical distribution of electrons in the molecule results in no net dipole moment, making CO a non-polar molecule.
We hope this explanation has helped clarify the properties of carbon monoxide and why it is not considered a polar molecule.
Share Your Thoughts
Do you have any questions about polar molecules or carbon monoxide? Share your thoughts in the comments below!



What is a polar molecule?
+A polar molecule is a molecule that has a net dipole moment, meaning it has a slightly positive charge on one end and a slightly negative charge on the other end.
Is CO a polar molecule?
+No, CO is not a polar molecule. While it has a slight imbalance of charge due to the difference in electronegativity between the carbon and oxygen atoms, it is still considered a non-polar molecule.
What is the difference between a polar and non-polar molecule?
+A polar molecule has a net dipole moment, while a non-polar molecule does not. Polar molecules have a slight imbalance of charge, while non-polar molecules have a symmetrical distribution of electrons.