The world of chemistry can be a complex and fascinating place, especially when it comes to chemical reactions. One crucial concept in understanding these reactions is identifying the limiting reactant and excess reactant. In this article, we will delve into the world of chemical reactions, explore the importance of identifying excess reactants, and provide a step-by-step guide on how to do so with ease.
Chemical reactions are the heart of chemistry, and understanding how they work is vital for any aspiring chemist. In a chemical reaction, reactants are converted into products, and the amount of each reactant used can affect the outcome of the reaction. In an ideal world, all reactants would be used up completely, leaving no excess. However, in reality, this is often not the case. One reactant may be used up completely, leaving others in excess.

The importance of identifying excess reactants cannot be overstated. In many chemical reactions, excess reactants can affect the yield of the desired product, and in some cases, can even be hazardous. For example, in the production of ammonia, excess nitrogen can lead to the formation of unwanted byproducts, reducing the overall yield of the reaction.
Understanding Limiting and Excess Reactants
To understand excess reactants, we first need to understand the concept of limiting reactants. A limiting reactant is the reactant that is completely used up in a chemical reaction, determining the amount of product formed. On the other hand, an excess reactant is the reactant that is not completely used up, leaving some of it remaining after the reaction is complete.
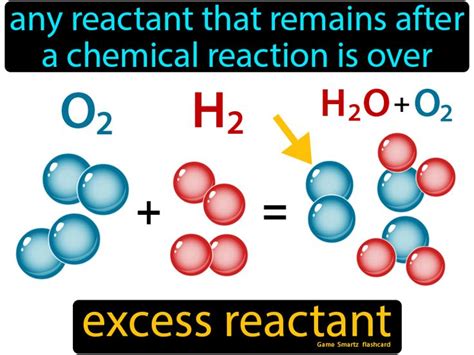
For example, consider the reaction between hydrogen and oxygen to form water:
2H2 + O2 → 2H2O
In this reaction, if we start with 2 moles of hydrogen and 1 mole of oxygen, the oxygen will be the limiting reactant, as it will be completely used up to form 2 moles of water. The excess reactant, in this case, will be the hydrogen, as there will be 1 mole of it left over after the reaction is complete.
How to Find Excess Reactant
Finding excess reactant in a chemical reaction involves a simple, step-by-step process. Here's how to do it:
- Write the balanced equation: The first step is to write the balanced equation for the reaction. This will give you the mole ratios of the reactants and products.
- Determine the number of moles: Determine the number of moles of each reactant present initially. This can be done using the molar mass of each reactant and the given mass.
- Calculate the mole ratio: Calculate the mole ratio of each reactant to the limiting reactant. This can be done by dividing the number of moles of each reactant by the number of moles of the limiting reactant.
- Compare the mole ratios: Compare the calculated mole ratios to the mole ratios in the balanced equation. If the calculated mole ratio is greater than the mole ratio in the balanced equation, then the reactant is in excess.

For example, consider the reaction between sodium and chlorine to form sodium chloride:
2Na + Cl2 → 2NaCl
If we start with 2 moles of sodium and 1.5 moles of chlorine, we can calculate the mole ratio of chlorine to sodium as follows:
Mole ratio of Cl2 to Na = 1.5 mol Cl2 / 2 mol Na = 0.75
Comparing this to the mole ratio in the balanced equation (1:2), we can see that chlorine is in excess.
Real-World Applications of Excess Reactant
Excess reactant plays a crucial role in many real-world applications, from industrial processes to everyday life. Here are a few examples:
- Ammonia production: In the production of ammonia, excess nitrogen can lead to the formation of unwanted byproducts, reducing the overall yield of the reaction.
- Pharmaceuticals: In the production of pharmaceuticals, excess reactants can lead to the formation of impurities, which can affect the efficacy and safety of the final product.
- Food processing: In food processing, excess reactants can lead to the formation of unwanted compounds, which can affect the flavor, texture, and nutritional value of the final product.
Common Mistakes to Avoid
When finding excess reactant, there are a few common mistakes to avoid:
- Not writing the balanced equation: Failing to write the balanced equation can lead to incorrect mole ratios and excess reactant calculations.
- Not calculating the mole ratio: Failing to calculate the mole ratio of each reactant can lead to incorrect excess reactant calculations.
- Not comparing the mole ratios: Failing to compare the calculated mole ratios to the mole ratios in the balanced equation can lead to incorrect excess reactant calculations.

Gallery of Printable Stoichiometry






FAQ Section
What is the difference between limiting and excess reactant?
+A limiting reactant is the reactant that is completely used up in a chemical reaction, determining the amount of product formed. An excess reactant is the reactant that is not completely used up, leaving some of it remaining after the reaction is complete.
How do I calculate the mole ratio of each reactant?
+The mole ratio of each reactant can be calculated by dividing the number of moles of each reactant by the number of moles of the limiting reactant.
What are some common mistakes to avoid when finding excess reactant?
+Common mistakes to avoid include not writing the balanced equation, not calculating the mole ratio, and not comparing the mole ratios to the mole ratios in the balanced equation.
In conclusion, finding excess reactant in chemical reactions is a crucial concept in chemistry that can be made easy by following a simple, step-by-step process. By understanding the concept of limiting and excess reactant, and by avoiding common mistakes, you can master the art of finding excess reactant and become a proficient chemist. Whether you're a student, a teacher, or a professional chemist, this article has provided you with the tools and knowledge to take your chemistry skills to the next level.