The world of chemistry and physics is filled with various units of measurement, each with its own significance and application. Among these, the gram-mole (g/mol) and dalton (Da) are two commonly used units in chemistry, particularly in the study of molecular weights and chemical reactions. However, converting between these units can be a bit confusing, especially for those new to the field. In this article, we will delve into the world of unit conversions, specifically focusing on how to convert g/mol to daltons.
Understanding the Basics: G/mol and Daltons
Before we dive into the conversion process, it's essential to understand what each unit represents.
- Gram-mole (g/mol): This unit is used to express the molecular weight of a substance. It represents the mass of one mole of a substance in grams.
- Dalton (Da): Named after John Dalton, the dalton is a unit of mass used to express the mass of atoms and molecules. It is defined as one-twelfth the mass of a carbon-12 atom.
The Conversion Factor: Avogadro's Number
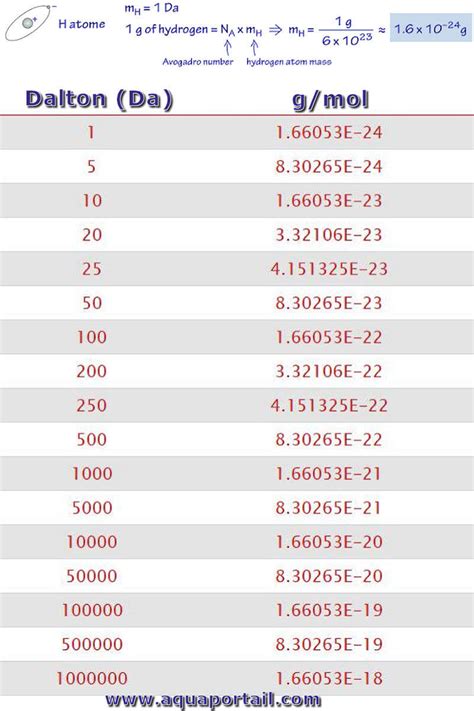
The key to converting g/mol to daltons lies in Avogadro's number, which is a fundamental constant in chemistry. Avogadro's number (6.022 x 10^23) represents the number of particles (atoms or molecules) in one mole of a substance.
Converting G/mol to Daltons: A Step-by-Step Guide
Now that we have a basic understanding of the units and the conversion factor, let's proceed with the conversion process.
-
Identify the molecular weight in g/mol: Start by identifying the molecular weight of the substance you want to convert. This value is usually expressed in g/mol.
-
Apply Avogadro's number: To convert the molecular weight from g/mol to daltons, divide the molecular weight by Avogadro's number (6.022 x 10^23).
Molecular weight (daltons) = Molecular weight (g/mol) / Avogadro's number
-
Perform the calculation: Plug in the values and perform the calculation.
Example: Convert the molecular weight of glucose (C6H12O6) from g/mol to daltons.
Molecular weight of glucose (g/mol) = 180.16 g/mol
Molecular weight (daltons) = 180.16 g/mol / (6.022 x 10^23)
Molecular weight (daltons) ≈ 180.16 Da
Practical Applications of G/mol to Dalton Conversions
Converting between g/mol and daltons has numerous practical applications in various fields, including:
- Biochemistry: Understanding the molecular weight of biomolecules, such as proteins and nucleic acids, is crucial in biochemistry. Converting between g/mol and daltons helps researchers calculate the molecular weight of these molecules.
- Pharmaceuticals: In the pharmaceutical industry, accurate molecular weight calculations are essential for drug development and quality control. Converting between g/mol and daltons ensures that molecular weights are expressed in the correct units.
Tips and Tricks for Accurate Conversions
To ensure accurate conversions, keep the following tips in mind:
- Use Avogadro's number correctly: Make sure to use the correct value of Avogadro's number (6.022 x 10^23) when performing conversions.
- Check your units: Verify that the units of the molecular weight are in g/mol before performing the conversion.
- Round correctly: Round your final answer to the correct number of significant figures.

Conclusion
Converting between g/mol and daltons is a crucial skill in chemistry, particularly in the study of molecular weights and chemical reactions. By understanding the basics of each unit and applying Avogadro's number, you can accurately convert between g/mol and daltons. Remember to use the correct conversion factor, check your units, and round correctly to ensure accurate results.






What is the difference between g/mol and daltons?
+G/mol (gram-mole) is a unit of molecular weight, while daltons (Da) are a unit of mass used to express the mass of atoms and molecules.
How do I convert g/mol to daltons?
+To convert g/mol to daltons, divide the molecular weight in g/mol by Avogadro's number (6.022 x 10^23).
What is Avogadro's number?
+Avogadro's number (6.022 x 10^23) is a fundamental constant in chemistry that represents the number of particles (atoms or molecules) in one mole of a substance.