Sodium sulfate, a naturally occurring mineral, has become an integral part of various industries, including detergents, paper manufacturing, and glass production. With its widespread use, it's essential to have a deep understanding of its chemical formula and properties. In this article, we'll delve into the sodium sulfate formula, exploring its composition, uses, and benefits.
What is the Sodium Sulfate Formula?
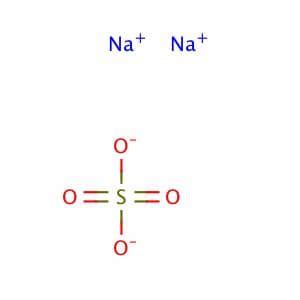
The sodium sulfate formula is Na2SO4. This compound consists of two sodium ions (Na+) and one sulfate ion (SO42-). The sulfate ion is composed of one sulfur atom bonded to four oxygen atoms. The sodium ions are bonded to the sulfate ion through ionic bonds, resulting in a neutral compound.

How is Sodium Sulfate Used?
Sodium sulfate has a wide range of applications across various industries. Some of its most notable uses include:
- Detergents and Cleaning Agents: Sodium sulfate is used as a filler in detergents, helping to improve their cleaning power and reduce costs.
- Paper Manufacturing: Sodium sulfate is used to produce paper pulp, which is then used to manufacture paper products such as packaging materials and printing papers.
- Glass Production: Sodium sulfate is used as a flux in glass production, helping to reduce the melting point of silica and other minerals.
Benefits of Sodium Sulfate
Sodium sulfate offers several benefits, making it a valuable compound in various industries. Some of its key benefits include:
- Cost-Effective: Sodium sulfate is a relatively inexpensive compound, making it an attractive option for industries looking to reduce costs.
- Environmentally Friendly: Sodium sulfate is a natural mineral that is biodegradable and non-toxic, making it a more environmentally friendly option compared to other compounds.
- Versatile: Sodium sulfate has a wide range of applications, making it a versatile compound that can be used in various industries.
Production of Sodium Sulfate
Sodium sulfate is produced through the reaction of sulfuric acid with sodium chloride (common table salt). This reaction is known as the Mannheim process. The reaction is as follows:
NaCl + H2SO4 → Na2SO4 + HCl
This reaction produces sodium sulfate and hydrochloric acid. The sodium sulfate is then crystallized and purified to produce a high-quality product.

Physical and Chemical Properties
Sodium sulfate has several physical and chemical properties that make it a valuable compound. Some of its key properties include:
- Appearance: Sodium sulfate is a white crystalline solid.
- Odor: Sodium sulfate is odorless.
- Solubility: Sodium sulfate is highly soluble in water.
- Melting Point: Sodium sulfate has a melting point of 884°C.
- Boiling Point: Sodium sulfate has a boiling point of 1429°C.

Applications of Sodium Sulfate
Sodium sulfate has a wide range of applications across various industries. Some of its most notable applications include:
- Textile Industry: Sodium sulfate is used as a dyeing agent in the textile industry.
- Paper Industry: Sodium sulfate is used to produce paper pulp, which is then used to manufacture paper products.
- Glass Industry: Sodium sulfate is used as a flux in glass production, helping to reduce the melting point of silica and other minerals.

Conclusion
Sodium sulfate is a naturally occurring mineral that has become an integral part of various industries. With its widespread use, it's essential to have a deep understanding of its chemical formula and properties. In this article, we've explored the sodium sulfate formula, its composition, uses, and benefits. We've also discussed its production, physical and chemical properties, and applications. As we continue to use sodium sulfate in various industries, it's essential to recognize its value and importance.

Gallery of Sodium Sulfate






What is the sodium sulfate formula?
+The sodium sulfate formula is Na2SO4.
What are the uses of sodium sulfate?
+Sodium sulfate is used in various industries, including detergents, paper manufacturing, and glass production.
What are the benefits of sodium sulfate?
+Sodium sulfate is cost-effective, environmentally friendly, and versatile, making it a valuable compound in various industries.
We hope you found this article informative and helpful. If you have any questions or comments, please feel free to share them below.