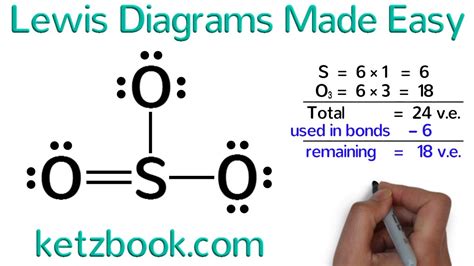
Understanding Lewis Dot Structure
The Lewis dot structure, also known as the Lewis electron dot structure, is a simplified way to represent the valence electrons of atoms within a molecule. It's a fundamental concept in chemistry that helps us visualize the bonding between atoms. In this article, we'll delve into the world of Lewis dot structures, exploring their importance, benefits, and a step-by-step guide on how to create them.

What is a Lewis Dot Structure?
A Lewis dot structure is a two-dimensional representation of a molecule, showing the arrangement of valence electrons around the atoms. It's a simple yet powerful tool that helps us understand the chemical bonding between atoms. The structure consists of dots, which represent the valence electrons, and lines, which represent the chemical bonds between atoms.
Benefits of Lewis Dot Structure
The Lewis dot structure has several benefits that make it an essential tool in chemistry:
- Simplifies complex molecular structures: By representing molecules in a two-dimensional format, Lewis dot structures make it easier to visualize and understand complex molecular structures.
- Helps predict chemical properties: By analyzing the Lewis dot structure of a molecule, we can predict its chemical properties, such as reactivity and polarity.
- Facilitates understanding of chemical bonding: The Lewis dot structure helps us understand the type of chemical bonds between atoms, including covalent, ionic, and metallic bonds.

Step-by-Step Guide to Creating Lewis Dot Structures
Creating Lewis dot structures is a straightforward process that involves the following steps:
- Determine the total number of valence electrons: Calculate the total number of valence electrons in the molecule by summing the valence electrons of each atom.
- Draw the skeletal structure: Draw the skeletal structure of the molecule, showing the arrangement of atoms.
- Distribute the electrons: Distribute the valence electrons around the atoms, following the octet rule.
- Form bonds: Form chemical bonds between atoms by sharing or transferring electrons.
Common Mistakes to Avoid
When creating Lewis dot structures, there are several common mistakes to avoid:
- Incorrectly counting valence electrons: Ensure that you accurately count the total number of valence electrons in the molecule.
- Misplacing electrons: Ensure that you place the electrons in the correct positions around the atoms.
- Forgetting to form bonds: Ensure that you form chemical bonds between atoms by sharing or transferring electrons.

Conclusion
In conclusion, Lewis dot structures are a powerful tool in chemistry that helps us visualize and understand the chemical bonding between atoms. By following the step-by-step guide outlined in this article, you can create accurate Lewis dot structures and avoid common mistakes.
Gallery of Lewis Dot Structures






Frequently Asked Questions
What is the purpose of Lewis dot structures?
+Lewis dot structures help us visualize and understand the chemical bonding between atoms in a molecule.
How do I create a Lewis dot structure?
+To create a Lewis dot structure, follow these steps: determine the total number of valence electrons, draw the skeletal structure, distribute the electrons, and form bonds.
What are some common mistakes to avoid when creating Lewis dot structures?
+Common mistakes to avoid include incorrectly counting valence electrons, misplacing electrons, and forgetting to form bonds.