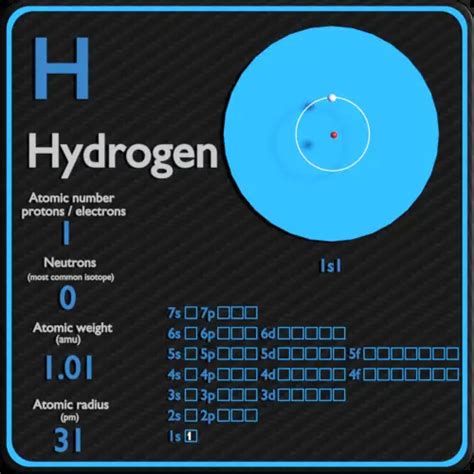
The periodic table is a fundamental tool in chemistry, organizing elements in a logical and systematic way. Each element has its unique properties, which set it apart from others. One such element is hydrogen, the lightest and most abundant element in the universe. Hydrogen's unique property of having the fewest electrons makes it an essential component of many molecules, including water, acids, and organic compounds.
What Makes Hydrogen So Special?
Hydrogen is the first element on the periodic table, with an atomic number of 1. This means it has only one proton in its atomic nucleus. As a result, hydrogen's atomic structure is incredibly simple, consisting of a single proton and a single electron. This simplicity makes hydrogen an extremely reactive element, capable of forming bonds with a wide range of other elements.
The Importance of Hydrogen's Electron Configuration
Hydrogen's electron configuration is 1s1, meaning its single electron occupies the 1s orbital. This electron configuration is crucial in understanding hydrogen's chemical properties. The single electron makes hydrogen highly reactive, as it is eager to form bonds with other elements to achieve a more stable electron configuration.
Hydrogen's Unique Properties in Molecules
Hydrogen's unique property of having the fewest electrons makes it an essential component of many molecules. In water (H2O), for example, two hydrogen atoms bond with a single oxygen atom, forming a covalent bond. This bond is responsible for water's unique properties, such as its high surface tension and boiling point.
In acids, hydrogen's reactivity allows it to form bonds with other elements, such as oxygen, nitrogen, and sulfur. This reactivity is essential for the formation of acids, which play a crucial role in many biological and chemical processes.
Practical Applications of Hydrogen's Unique Properties
Hydrogen's unique properties have numerous practical applications in various fields. In the field of energy, hydrogen is being explored as a clean-burning fuel, which could potentially replace fossil fuels. Hydrogen fuel cells, for example, use hydrogen to generate electricity, producing only water and heat as byproducts.
In the field of chemistry, hydrogen's reactivity makes it an essential component in many chemical reactions. Hydrogenation reactions, for example, use hydrogen to add hydrogen atoms to unsaturated compounds, resulting in the formation of saturated compounds.
Conclusion
In conclusion, hydrogen's unique property of having the fewest electrons makes it an essential component of many molecules. Its simplicity and reactivity make it a crucial element in many biological and chemical processes. As research continues to explore the properties and applications of hydrogen, its importance in various fields will only continue to grow.

Benefits of Hydrogen's Unique Properties
- Highly reactive, allowing it to form bonds with a wide range of elements
- Essential component of many molecules, including water, acids, and organic compounds
- Potential clean-burning fuel, which could replace fossil fuels
- Crucial element in many biological and chemical processes
Working Mechanisms of Hydrogen's Unique Properties
- Hydrogen's simple atomic structure, consisting of a single proton and a single electron
- Hydrogen's electron configuration, 1s1, which makes it highly reactive
- Hydrogen's ability to form covalent bonds with other elements

Steps to Utilize Hydrogen's Unique Properties
- Understand the properties and applications of hydrogen
- Explore the use of hydrogen as a clean-burning fuel
- Study the role of hydrogen in biological and chemical processes
- Develop new technologies that utilize hydrogen's unique properties
Gallery of Hydrogen-Related Images





Frequently Asked Questions
What is the atomic number of hydrogen?
+The atomic number of hydrogen is 1.
What is the electron configuration of hydrogen?
+The electron configuration of hydrogen is 1s1.
What are some practical applications of hydrogen's unique properties?
+Hydrogen's unique properties have numerous practical applications in various fields, including energy, chemistry, and biology.
We hope you have enjoyed this article on hydrogen's unique property of having the fewest electrons. Share your thoughts and comments below!