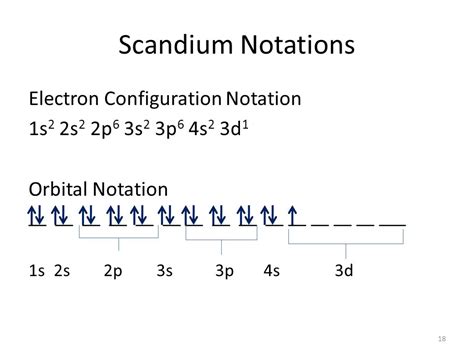
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons in an atom. Mastering electron configuration is crucial for understanding various chemical properties and phenomena. Scandium, a transition metal with the atomic number 21, has a unique electron configuration that sets it apart from other elements. In this article, we will explore five ways to master electron configuration for scandium.
Understanding the Basics of Electron Configuration
Before diving into the specifics of scandium's electron configuration, it's essential to understand the basics. Electron configuration refers to the arrangement of electrons in an atom, which is determined by the energy levels or shells that electrons occupy. The energy levels are divided into subshells, which are further divided into orbitals. Each orbital can hold a specific number of electrons, and the electrons in an atom occupy the lowest available energy levels.

1. Learn the Aufbau Principle and the Periodic Table
The Aufbau principle states that electrons occupy the lowest available energy levels in an atom. This principle is essential for understanding electron configuration. By studying the periodic table, you can identify the energy levels and subshells that electrons occupy in an atom. Scandium, with its atomic number 21, has an electron configuration of [Ar] 3d1 4s2.

2. Understand the Role of Electrons in Shells and Subshells
Electrons in an atom occupy specific shells and subshells, which are determined by the energy levels. The first shell has one subshell (1s), the second shell has two subshells (2s and 2p), and the third shell has three subshells (3s, 3p, and 3d). Scandium's electron configuration shows that its electrons occupy the 3d and 4s subshells.

3. Apply the Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This principle is essential for understanding electron configuration. By applying the Pauli exclusion principle, you can determine the electron configuration of scandium and other elements.

4. Practice Writing Electron Configurations
Practice is key to mastering electron configuration. By writing electron configurations for various elements, including scandium, you can develop a deeper understanding of the concept. Start with simple elements and gradually move to more complex ones.

5. Use Online Resources and Visual Aids
Online resources and visual aids can be incredibly helpful in mastering electron configuration. Websites such as WebElements and Electron Configuration Calculator provide interactive tools and diagrams to help you understand electron configuration. Visual aids such as electron configuration diagrams and periodic tables can also help you visualize the concept.

Gallery of Electron Configuration Diagrams






FAQs
What is electron configuration?
+Electron configuration is the arrangement of electrons in an atom, which is determined by the energy levels or shells that electrons occupy.
What is the Aufbau principle?
+The Aufbau principle states that electrons occupy the lowest available energy levels in an atom.
How do I practice writing electron configurations?
+Start with simple elements and gradually move to more complex ones. Use online resources and visual aids to help you understand the concept.
In conclusion, mastering electron configuration for scandium requires a deep understanding of the concept and its application. By following the five ways outlined in this article, you can develop a strong foundation in electron configuration and improve your understanding of chemistry. Remember to practice regularly and use online resources and visual aids to help you master the concept.