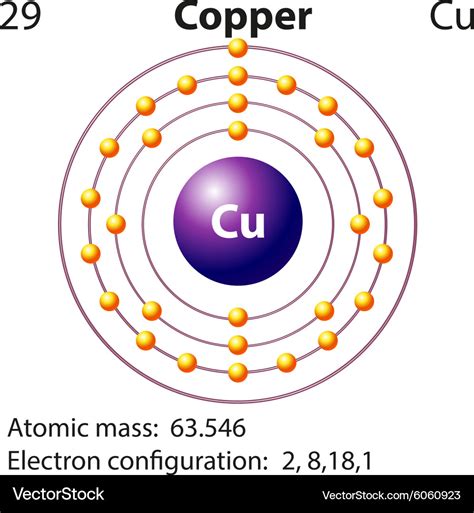
The periodic table is a powerful tool that helps us understand the properties and behavior of elements. One of the key concepts in understanding the periodic table is electron configuration, which describes the arrangement of electrons in an atom. In this article, we will explore the electron configuration of copper, a transition metal with the symbol Cu and atomic number 29.
What is Electron Configuration?
Electron configuration is a way of describing the arrangement of electrons in an atom. It is a fundamental concept in chemistry and physics that helps us understand the properties and behavior of elements. Electron configuration is based on the idea that electrons occupy specific energy levels or orbitals around the nucleus of an atom.
The Aufbau Principle and Hund's Rule
To understand the electron configuration of copper, we need to understand two important principles: the Aufbau principle and Hund's rule. The Aufbau principle states that electrons occupy the lowest available energy levels in an atom. Hund's rule states that when filling orbitals of equal energy, electrons occupy them singly and with parallel spins before pairing up.
The Electron Configuration of Copper
The electron configuration of copper is [Ar] 3d10 4s1. This means that the outermost energy level of a copper atom has one electron in the s-orbital and ten electrons in the d-orbitals. The [Ar] notation indicates that the inner energy levels are similar to those of argon, a noble gas with a full outer energy level.

Understanding the 3d and 4s Orbitals
The 3d and 4s orbitals are important in understanding the electron configuration of copper. The 3d orbitals are a set of five degenerate orbitals that are similar in energy. The 4s orbital is a single orbital that is higher in energy than the 3d orbitals. In copper, the 3d orbitals are fully occupied, and the 4s orbital has one electron.
Why is Copper's Electron Configuration Important?
Copper's electron configuration is important because it helps us understand its properties and behavior. Copper is a transition metal with a number of unique properties, including its high electrical conductivity, thermal conductivity, and ductility. These properties make copper an important material in a wide range of applications, including electrical wiring, electronics, and architecture.
Uses of Copper**
Copper has a number of important uses due to its unique properties. Some of the most significant uses of copper include:
- Electrical Wiring: Copper is an excellent conductor of electricity, making it a popular choice for electrical wiring.
- Electronics: Copper is used extensively in electronics, including in printed circuit boards, switches, and connectors.
- Architecture: Copper is used in architecture due to its attractive appearance and durability. It is often used for roofing, cladding, and other decorative features.

Conclusion
In conclusion, the electron configuration of copper is an important concept that helps us understand its properties and behavior. Copper's unique electron configuration, with its fully occupied 3d orbitals and single electron in the 4s orbital, makes it an important material in a wide range of applications. Whether you're an engineer, architect, or simply someone interested in science, understanding the electron configuration of copper can help you appreciate its importance and versatility.
Gallery of Copper Electron Configuration






Frequently Asked Questions
What is the electron configuration of copper?
+The electron configuration of copper is [Ar] 3d10 4s1.
Why is copper's electron configuration important?
+Copper's electron configuration is important because it helps us understand its properties and behavior.
What are some common uses of copper?
+Copper is used extensively in electrical wiring, electronics, and architecture.