The world of chemistry can be fascinating, yet overwhelming, especially when it comes to understanding the intricacies of atomic structures. One fundamental concept that helps us grasp the properties and behavior of elements is electron configuration. In this article, we'll delve into the electron configuration of copper, a vital element in various industries, and explore its significance in a simple and engaging manner.
What is Electron Configuration?
Electron configuration refers to the arrangement of electrons within an atom, specifically in the orbitals surrounding the nucleus. It's a way to describe how electrons are distributed among the various energy levels or shells, which is crucial in understanding an element's chemical properties. Think of it like a game of Tetris, where electrons occupy specific positions in the atomic structure.
The Electron Configuration of Copper
Copper is a transition metal with the atomic number 29, meaning it has 29 protons in its nucleus. To write the electron configuration of copper, we need to consider the Aufbau principle and the Pauli exclusion principle.
The Aufbau principle states that electrons occupy the lowest available energy levels, while the Pauli exclusion principle dictates that each orbital can hold a maximum of two electrons with opposite spins. Using these principles, we can construct the electron configuration of copper as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹
Let's break down this configuration:
- The first energy level (1s) contains two electrons.
- The second energy level (2s and 2p) is filled with eight electrons (two in 2s and six in 2p).
- The third energy level (3s and 3p) is also filled with eight electrons (two in 3s and six in 3p).
- The 3d subshell, which is part of the third energy level, contains ten electrons.
- The fourth energy level (4s) contains one electron.
Visualizing the Electron Configuration of Copper
To better understand the electron configuration of copper, let's visualize it using a diagram.

In this diagram, each energy level is represented by a circle, and the electrons are depicted as small arrows. The 1s, 2s, and 3s subshells are filled with two electrons each, while the 2p and 3p subshells contain six electrons each. The 3d subshell is filled with ten electrons, and the 4s subshell contains one electron.
Significance of Copper's Electron Configuration
The electron configuration of copper plays a crucial role in determining its chemical properties. For example:
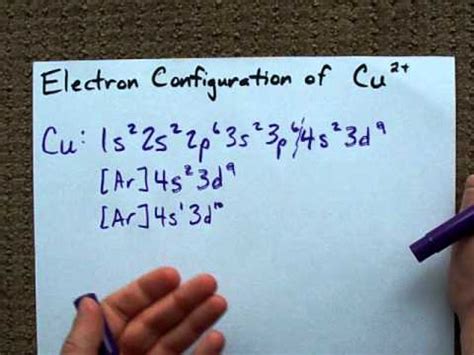
- Copper's electron configuration explains its ability to form ions with a +2 charge (Cu²⁺). This is because the 4s electron is easily lost, leaving a full 3d subshell and a +2 charge.
- The filled 3d subshell contributes to copper's high melting and boiling points, as well as its high density.
- Copper's electron configuration also influences its reactivity, particularly its ability to form compounds with other elements.
Practical Applications of Copper's Electron Configuration
Understanding the electron configuration of copper has numerous practical applications in various industries, including:
- Electrical engineering: Copper's high electrical conductivity is due to its electron configuration, making it an ideal material for electrical wiring and circuits.
- Architecture: Copper's corrosion resistance and durability make it a popular choice for building materials, such as roofing and plumbing.
- Electronics: Copper's high thermal conductivity and ability to form compounds with other elements make it a crucial component in electronic devices, such as heat sinks and connectors.
Gallery of Copper Electron Configuration






FAQs
What is the electron configuration of copper?
+The electron configuration of copper is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹.
Why is copper's electron configuration important?
+Copper's electron configuration plays a crucial role in determining its chemical properties, such as its ability to form ions and compounds with other elements.
What are some practical applications of copper's electron configuration?
+Copper's electron configuration has numerous practical applications in various industries, including electrical engineering, architecture, and electronics.
Conclusion
In conclusion, understanding the electron configuration of copper is essential in grasping its chemical properties and behavior. The unique arrangement of electrons in copper's atomic structure contributes to its high melting and boiling points, corrosion resistance, and ability to form compounds with other elements. By exploring the electron configuration of copper, we can appreciate the significance of this element in various industries and its impact on our daily lives.