Lithium is a highly reactive alkali metal that is often used in batteries and other electronic devices due to its unique properties. One of the key characteristics of lithium is its ability to conduct electricity. But how well does lithium conduct electricity, and what makes it useful for this purpose?

What is Lithium?
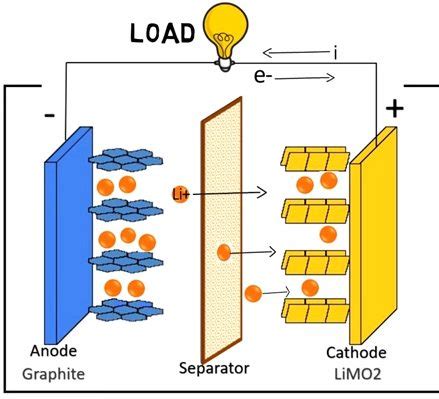
Lithium is the lightest of all metals and has an atomic number of 3. It is a soft, silvery-white metal that is highly reactive and has a low melting point. Lithium is often used in batteries, particularly lithium-ion batteries, which are commonly used in portable electronic devices such as smartphones and laptops.
Why is Lithium Used in Batteries?
Lithium is used in batteries because of its unique properties, which make it an ideal material for this application. Some of the key reasons why lithium is used in batteries include:
- High Energy Density: Lithium has a high energy density, which means that it can store a lot of energy relative to its size and weight.
- High Discharge Rate: Lithium can discharge quickly, which makes it useful for applications where a high burst of energy is required.
- Low Self-Discharge Rate: Lithium has a low self-discharge rate, which means that it can retain its charge for a long time.

Does Lithium Conduct Electricity Well?
Lithium is a good conductor of electricity, but it is not as good as some other metals. The conductivity of lithium is due to its high electron mobility, which means that its electrons are highly mobile and can move freely through the material.
The conductivity of lithium is also affected by its purity and the presence of impurities. Pure lithium is a good conductor of electricity, but the presence of impurities can reduce its conductivity.
Comparison with Other Metals
The conductivity of lithium is compared to other metals in the following table:
| Metal | Conductivity (S/m) |
|---|---|
| Copper | 5.96 x 10^7 |
| Aluminum | 3.45 x 10^7 |
| Silver | 6.30 x 10^7 |
| Lithium | 3.98 x 10^6 |
As can be seen from the table, lithium is not as good a conductor of electricity as some other metals, such as copper, aluminum, and silver. However, its unique properties and high energy density make it a useful material for batteries and other electronic devices.

Conclusion
In conclusion, lithium is a good conductor of electricity, but it is not as good as some other metals. Its unique properties and high energy density make it a useful material for batteries and other electronic devices. The conductivity of lithium is affected by its purity and the presence of impurities, and it is often used in combination with other materials to improve its conductivity.
We hope this article has provided you with a better understanding of lithium and its properties. If you have any questions or comments, please feel free to ask.





What is lithium used for?
+Lithium is used in batteries, particularly lithium-ion batteries, which are commonly used in portable electronic devices such as smartphones and laptops.
Is lithium a good conductor of electricity?
+Lithium is a good conductor of electricity, but it is not as good as some other metals, such as copper, aluminum, and silver.
What affects the conductivity of lithium?
+The conductivity of lithium is affected by its purity and the presence of impurities.