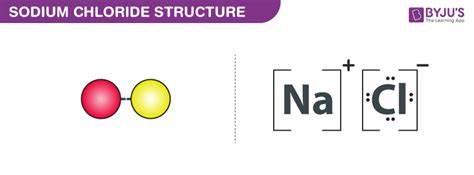
Sodium (Na) and chlorine (Cl) are two elements that are commonly found in nature and are essential for various biological and industrial processes. When combined, they form a compound known as sodium chloride, or table salt, which is a vital component of our diets. However, the question remains: do Na and Cl bond, and if so, what are the key things to know about this bonding process?
In this article, we will delve into the world of chemistry and explore the fascinating world of bonding between sodium and chlorine. We will examine the key things to know about this bonding process, including the types of bonds formed, the properties of sodium chloride, and the uses of this compound in various industries.
What is the Bonding Process Between Na and Cl?
When sodium and chlorine combine, they form a strong ionic bond. This type of bond is formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. In the case of sodium and chlorine, the sodium atom loses one electron to form a positively charged ion (Na+), while the chlorine atom gains one electron to form a negatively charged ion (Cl-).
The electrostatic attraction between the positively charged sodium ion and the negatively charged chlorine ion leads to the formation of a strong ionic bond. This bond is responsible for the stability and properties of sodium chloride.

Types of Bonds Formed Between Na and Cl
The bonding process between sodium and chlorine results in the formation of a single type of bond, namely an ionic bond. However, it is worth noting that sodium chloride can also exhibit other types of bonds, such as covalent bonds, in certain situations.
For example, when sodium chloride is dissolved in water, the ionic bonds between the sodium and chlorine ions are broken, and the ions are free to move and interact with other molecules. In this case, the sodium and chlorine ions can form weak covalent bonds with water molecules, which are responsible for the solubility of sodium chloride in water.
Properties of Sodium Chloride
Sodium chloride, or table salt, is a compound with a wide range of properties that make it an essential component of our diets and various industrial processes. Some of the key properties of sodium chloride include:
- High melting point: Sodium chloride has a high melting point of 801°C, which makes it useful in various industrial applications, such as the production of glass and ceramics.
- High boiling point: Sodium chloride has a high boiling point of 1413°C, which makes it useful in various industrial applications, such as the production of textiles and paper.
- Solubility in water: Sodium chloride is highly soluble in water, which makes it useful in various applications, such as cooking and cleaning.
- Conductivity: Sodium chloride is an excellent conductor of electricity, which makes it useful in various industrial applications, such as the production of batteries and electronics.

Uses of Sodium Chloride
Sodium chloride is a compound with a wide range of uses in various industries. Some of the key uses of sodium chloride include:
- Food industry: Sodium chloride is a vital component of our diets, and is used as a seasoning and preservative in various food products.
- Industrial applications: Sodium chloride is used in various industrial applications, such as the production of glass, ceramics, textiles, and paper.
- Cleaning products: Sodium chloride is used in various cleaning products, such as detergents and disinfectants.
- Medical applications: Sodium chloride is used in various medical applications, such as the treatment of dehydration and the production of medical equipment.
Conclusion
In conclusion, the bonding process between sodium and chlorine is a complex and fascinating process that results in the formation of a strong ionic bond. This bond is responsible for the stability and properties of sodium chloride, which is a compound with a wide range of uses in various industries. By understanding the key things to know about this bonding process, we can appreciate the importance of sodium chloride in our daily lives and the various industries that rely on it.






What is the bonding process between sodium and chlorine?
+The bonding process between sodium and chlorine is a complex process that results in the formation of a strong ionic bond. This bond is responsible for the stability and properties of sodium chloride.
What are the key properties of sodium chloride?
+Sodium chloride has a high melting point, high boiling point, and is highly soluble in water. It is also an excellent conductor of electricity.
What are the uses of sodium chloride?
+Sodium chloride is used in various industries, including the food industry, industrial applications, cleaning products, and medical applications.