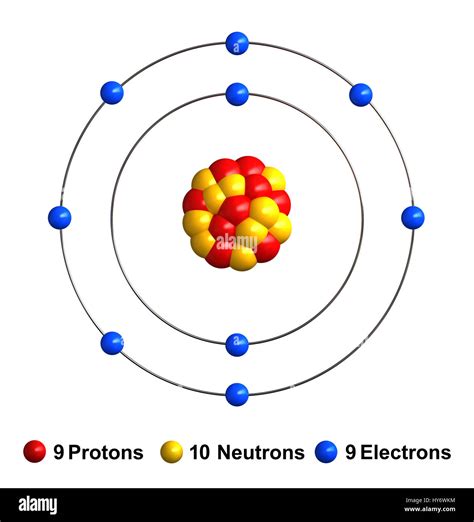
The Bohr model of an atom, proposed by Niels Bohr in 1913, is a simplified representation of the atomic structure that describes the arrangement of electrons around the nucleus. In this article, we will delve into the Bohr model of a fluorine atom, exploring its electron configuration, energy levels, and other key aspects.
Understanding the Bohr Model

The Bohr model depicts an atom as a small, heavy nucleus surrounded by electrons orbiting around it in fixed energy levels or shells. Each energy level has a specific capacity, and electrons fill these levels in a specific order. The model assumes that electrons occupy specific positions around the nucleus, which is not entirely accurate but provides a useful simplification for understanding atomic structure.
Fluorine Atom Electron Configuration

A fluorine atom has an atomic number of 9, which means it has 9 electrons. The electron configuration of fluorine is 1s² 2s² 2p⁵. This configuration indicates that:
- The first energy level (n = 1) contains two electrons in the 1s orbital.
- The second energy level (n = 2) contains two electrons in the 2s orbital and five electrons in the 2p orbitals.
Energy Levels and Electron Shells

The Bohr model describes the energy levels or shells around the nucleus as follows:
- The first energy level (n = 1) has one shell and can hold up to 2 electrons.
- The second energy level (n = 2) has two shells (s and p) and can hold up to 8 electrons.
- Each energy level has a specific capacity, and electrons fill these levels in a specific order.
Ionization Energy and Electron Affinity

Ionization energy is the energy required to remove an electron from a fluorine atom, while electron affinity is the energy released when an electron is added to a fluorine atom. Fluorine has a high ionization energy and a high electron affinity, indicating that it is highly reactive and tends to gain electrons to form a stable anion.
Limitations of the Bohr Model

While the Bohr model provides a useful simplification of the atomic structure, it has several limitations:
- The model assumes that electrons occupy specific positions around the nucleus, which is not entirely accurate.
- The model does not account for the wave-particle duality of electrons.
- The model is not applicable to atoms with more than one electron.
Modern Atomic Models

Modern atomic models, such as the quantum mechanical model and the atomic orbital model, provide a more accurate and detailed description of the atomic structure. These models account for the wave-particle duality of electrons and provide a more comprehensive understanding of atomic behavior.






What is the electron configuration of a fluorine atom?
+The electron configuration of a fluorine atom is 1s² 2s² 2p⁵.
What is the ionization energy of a fluorine atom?
+The ionization energy of a fluorine atom is 1681 kJ/mol.
What is the electron affinity of a fluorine atom?
+The electron affinity of a fluorine atom is 328 kJ/mol.