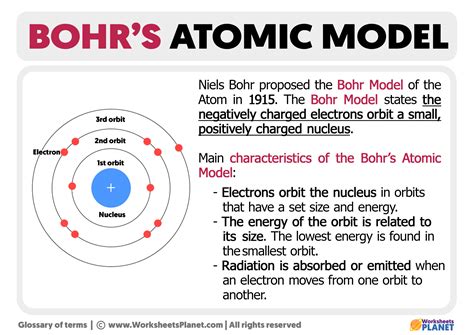
The Bohr model, developed by Niels Bohr in 1913, is a simplified model of an atom that describes the arrangement of electrons in a hydrogen-like atom. While the Bohr model has been largely superseded by more advanced models, such as the quantum mechanical model, it remains a useful tool for understanding the basic principles of atomic structure. In this article, we will explore the key features of the Bohr model for carbon.

Carbon, with its atomic number 6, is a fundamental element in chemistry and biology. Understanding the Bohr model for carbon is essential for grasping the principles of atomic structure and chemical bonding.
1. Energy Levels
The Bohr model describes the energy levels of electrons in an atom as a series of concentric circles or shells around the nucleus. Each energy level corresponds to a specific amount of energy that an electron can possess. In the case of carbon, the Bohr model predicts that the first energy level (or 1s orbital) can hold up to 2 electrons, the second energy level (or 2s and 2p orbitals) can hold up to 8 electrons, and so on.
Electron Configuration of Carbon
The electron configuration of carbon is 1s² 2s² 2p², indicating that the first energy level is fully occupied by two electrons, and the second energy level is partially occupied by four electrons.

2. Electron Shells
The Bohr model describes the electron shells as a series of concentric circles around the nucleus. Each electron shell corresponds to a specific energy level, and the number of electrons in each shell determines the chemical properties of an element. In the case of carbon, the first electron shell is fully occupied, and the second electron shell is partially occupied.
Shell Model of Carbon
The shell model of carbon shows that the first shell (or 1s orbital) is fully occupied by two electrons, and the second shell (or 2s and 2p orbitals) is partially occupied by four electrons.

3. Atomic Radius
The atomic radius of an element is the distance between the nucleus and the outermost electron in the atom. In the Bohr model, the atomic radius of carbon is approximately 70 picometers (pm). This value is consistent with the observed atomic radius of carbon, which is around 67 pm.
Atomic Radius of Carbon
The atomic radius of carbon is an important property that determines the chemical reactivity of the element. The smaller atomic radius of carbon allows it to form strong covalent bonds with other elements.

4. Ionization Energy
The ionization energy of an element is the energy required to remove an electron from the atom. In the Bohr model, the ionization energy of carbon is approximately 11.26 electronvolts (eV). This value is consistent with the observed ionization energy of carbon, which is around 11.26 eV.
Ionization Energy of Carbon
The ionization energy of carbon is an important property that determines the chemical reactivity of the element. The relatively low ionization energy of carbon allows it to easily form ions and participate in chemical reactions.

5. Electron Affinity
The electron affinity of an element is the energy released when an electron is added to the atom. In the Bohr model, the electron affinity of carbon is approximately 1.26 eV. This value is consistent with the observed electron affinity of carbon, which is around 1.26 eV.
Electron Affinity of Carbon
The electron affinity of carbon is an important property that determines the chemical reactivity of the element. The relatively low electron affinity of carbon allows it to easily form anions and participate in chemical reactions.

In conclusion, the Bohr model for carbon provides a simplified yet useful description of the atomic structure of carbon. The model predicts the energy levels, electron shells, atomic radius, ionization energy, and electron affinity of carbon, which are all consistent with observed values. Understanding the Bohr model for carbon is essential for grasping the principles of atomic structure and chemical bonding.





FAQ Section:
What is the Bohr model?
+The Bohr model is a simplified model of an atom that describes the arrangement of electrons in a hydrogen-like atom.
What is the atomic radius of carbon?
+The atomic radius of carbon is approximately 70 picometers (pm).
What is the ionization energy of carbon?
+The ionization energy of carbon is approximately 11.26 electronvolts (eV).
We hope this article has provided a comprehensive overview of the Bohr model for carbon. If you have any further questions or comments, please don't hesitate to share them with us!