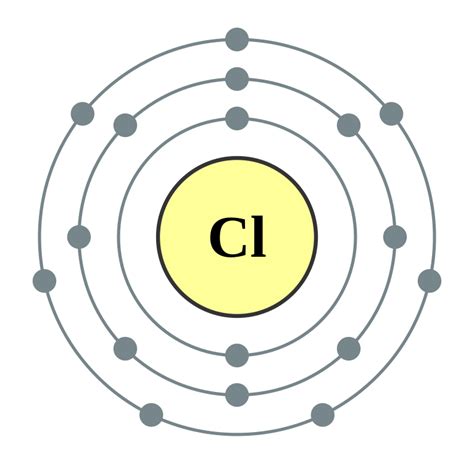
Chlorine, a halogen with a wide range of applications, is a fascinating element that can be better understood through the Bohr model diagram. This diagram, developed by Niels Bohr, is a simplified representation of an atom's structure, showing the relationships between the nucleus, electrons, and energy levels. In this article, we will delve into three key insights that the Bohr model diagram provides about chlorine, helping us unlock its secrets and understand its properties and behavior.
The Importance of Visualizing Atomic Structure
Before we dive into the specifics of chlorine's Bohr model diagram, it's essential to understand the significance of visualizing atomic structure. The Bohr model diagram provides a clear and concise representation of an atom's components, making it easier to comprehend complex concepts, such as electron configuration and energy levels. By visualizing the atomic structure, scientists and students can gain a deeper understanding of the element's properties and behavior, which is crucial for various applications, including chemistry, physics, and materials science.

Insight 1: Electron Configuration and Energy Levels
The Bohr model diagram of chlorine reveals its electron configuration, which is crucial for understanding its chemical properties and behavior. Chlorine's atomic number is 17, which means it has 17 electrons. The diagram shows that the electrons are arranged in three energy levels: the first energy level has two electrons, the second energy level has eight electrons, and the third energy level has seven electrons. This electron configuration is essential for understanding chlorine's reactivity and its ability to form compounds with other elements.

Insight 2: Valence Electrons and Reactivity
The Bohr model diagram of chlorine also highlights its valence electrons, which are the electrons in the outermost energy level. Chlorine has seven valence electrons, which makes it highly reactive. The diagram shows that the valence electrons are arranged in a specific pattern, with three pairs of electrons and one lone electron. This arrangement is responsible for chlorine's ability to form compounds with other elements, such as sodium and oxygen.

Insight 3: Ionization Energy and Electron Affinity
The Bohr model diagram of chlorine provides insight into its ionization energy and electron affinity, which are essential for understanding its chemical properties and behavior. Ionization energy is the energy required to remove an electron from an atom, while electron affinity is the energy released when an electron is added to an atom. The diagram shows that chlorine has a relatively high ionization energy and a relatively low electron affinity, which makes it more likely to gain electrons than lose them.

Gallery of Chlorine Diagrams






Frequently Asked Questions
What is the atomic number of chlorine?
+The atomic number of chlorine is 17.
What is the electron configuration of chlorine?
+The electron configuration of chlorine is 1s² 2s² 2p⁶ 3s² 3p⁵.
What is the valence electron configuration of chlorine?
+The valence electron configuration of chlorine is 3s² 3p⁵.
In conclusion, the Bohr model diagram of chlorine provides valuable insights into its atomic structure, electron configuration, and chemical properties. By understanding these insights, scientists and students can gain a deeper understanding of chlorine's behavior and applications. Whether you're a chemistry enthusiast or a student looking to improve your understanding of atomic structure, the Bohr model diagram of chlorine is an essential tool for unlocking the secrets of this fascinating element.