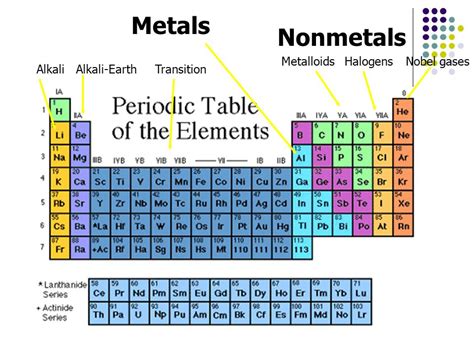
Nonmetals and noble gases are two distinct categories of elements in the periodic table, each with unique properties and characteristics. While both nonmetals and noble gases share some similarities, they are not the same thing.
In this article, we will delve into the world of chemistry and explore the differences between nonmetals and noble gases. We will examine their properties, electron configurations, and reactivity to gain a deeper understanding of these elements.
What are Nonmetals?
Nonmetals are a class of elements that do not exhibit the typical properties of metals, such as malleability, ductility, and the ability to conduct electricity. Nonmetals are typically found in the upper right-hand corner of the periodic table and include elements such as carbon, nitrogen, oxygen, and sulfur.
Nonmetals have a number of distinct properties, including:
- They are typically brittle and non-conductive
- They have a high electronegativity, meaning they tend to attract electrons
- They tend to form anions, or negatively charged ions
- They do not typically exhibit the same level of reactivity as metals

What are Noble Gases?
Noble gases, also known as inert gases, are a group of elements that are found in the far right-hand column of the periodic table. These elements are known for their stability and lack of reactivity, and include elements such as helium, neon, argon, krypton, xenon, and radon.
Noble gases have a number of distinct properties, including:
- They are highly unreactive, meaning they do not tend to form compounds with other elements
- They have a full outer energy level, meaning they do not tend to gain or lose electrons
- They are typically monatomic, meaning they exist as single atoms rather than molecules
- They are highly stable and do not tend to undergo chemical reactions

Key Differences between Nonmetals and Noble Gases
While nonmetals and noble gases share some similarities, there are a number of key differences between the two categories. Some of the main differences include:
- Reactivity: Nonmetals tend to be more reactive than noble gases, meaning they are more likely to form compounds with other elements.
- Electron configuration: Nonmetals tend to have a partially filled outer energy level, meaning they tend to gain or lose electrons. Noble gases, on the other hand, have a full outer energy level, meaning they do not tend to gain or lose electrons.
- Physical properties: Nonmetals tend to be brittle and non-conductive, while noble gases are typically monatomic and highly stable.
Physical Properties of Nonmetals and Noble Gases
Both nonmetals and noble gases have a number of distinct physical properties that set them apart from other elements.
Physical Properties of Nonmetals
Nonmetals tend to have a number of physical properties that are distinct from those of metals. Some of the main physical properties of nonmetals include:
- Brittleness: Nonmetals tend to be brittle, meaning they are prone to breaking or shattering when subjected to stress.
- Non-conductivity: Nonmetals tend to be non-conductive, meaning they do not tend to conduct electricity.
- Low melting points: Nonmetals tend to have low melting points, meaning they tend to melt at relatively low temperatures.

Physical Properties of Noble Gases
Noble gases tend to have a number of physical properties that are distinct from those of other elements. Some of the main physical properties of noble gases include:
- Monatomicity: Noble gases tend to be monatomic, meaning they exist as single atoms rather than molecules.
- High stability: Noble gases tend to be highly stable, meaning they do not tend to undergo chemical reactions.
- Low reactivity: Noble gases tend to be highly unreactive, meaning they do not tend to form compounds with other elements.

Chemical Properties of Nonmetals and Noble Gases
Both nonmetals and noble gases have a number of distinct chemical properties that set them apart from other elements.
Chemical Properties of Nonmetals
Nonmetals tend to have a number of chemical properties that are distinct from those of metals. Some of the main chemical properties of nonmetals include:
- Tendency to form anions: Nonmetals tend to form anions, or negatively charged ions.
- Tendency to form covalent bonds: Nonmetals tend to form covalent bonds, meaning they share electrons with other atoms.
- Tendency to undergo chemical reactions: Nonmetals tend to undergo chemical reactions, meaning they tend to form compounds with other elements.

Chemical Properties of Noble Gases
Noble gases tend to have a number of chemical properties that are distinct from those of other elements. Some of the main chemical properties of noble gases include:
- High stability: Noble gases tend to be highly stable, meaning they do not tend to undergo chemical reactions.
- Low reactivity: Noble gases tend to be highly unreactive, meaning they do not tend to form compounds with other elements.
- Tendency to remain uncombined: Noble gases tend to remain uncombined, meaning they do not tend to form compounds with other elements.

Gallery of Nonmetals and Noble Gases






Frequently Asked Questions
What is the main difference between nonmetals and noble gases?
+The main difference between nonmetals and noble gases is their reactivity. Nonmetals tend to be more reactive than noble gases, meaning they are more likely to form compounds with other elements.
What are some examples of nonmetals?
+Some examples of nonmetals include carbon, nitrogen, oxygen, and sulfur.
What are some examples of noble gases?
+Some examples of noble gases include helium, neon, argon, krypton, xenon, and radon.
In conclusion, nonmetals and noble gases are two distinct categories of elements with unique properties and characteristics. While nonmetals tend to be more reactive and form compounds with other elements, noble gases are highly stable and tend to remain uncombined. By understanding the differences between nonmetals and noble gases, we can gain a deeper appreciation for the diversity of elements in the periodic table.