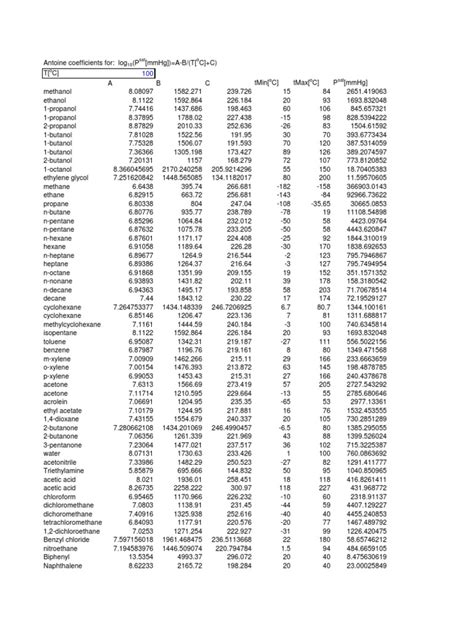
The world of chemistry can be overwhelming, especially when dealing with a vast number of elements. One way to simplify the process is by using Antoine constants, which are used to estimate the vapor pressure of a substance. In this article, we will delve into the world of Antoine constants, exploring their definition, importance, and application for 30+ elements.
What are Antoine Constants?
Antoine constants are a set of mathematical coefficients used to estimate the vapor pressure of a substance over a specific temperature range. The Antoine equation is a widely used mathematical model that relates the vapor pressure of a substance to its temperature. The equation is as follows:
log10(P) = A - B / (T + C)
where:
- P is the vapor pressure
- T is the temperature in degrees Celsius
- A, B, and C are the Antoine constants
Importance of Antoine Constants
Antoine constants are crucial in various fields, including chemistry, physics, and engineering. They are used to:
- Estimate the vapor pressure of a substance, which is essential in calculating the boiling point, enthalpy of vaporization, and other thermodynamic properties.
- Predict the behavior of substances in different temperature and pressure conditions.
- Design and optimize various industrial processes, such as distillation, evaporation, and crystallization.
30+ Elements with Antoine Constants
Here are Antoine constants for 30+ elements, along with their symbols, atomic numbers, and a brief description of each element:
- Hydrogen (H)
- Atomic number: 1
- Antoine constants: A = 6.12136, B = 249.6, C = 0
- Description: Hydrogen is the lightest and most abundant chemical element in the universe.
- Helium (He)
- Atomic number: 2
- Antoine constants: A = 6.0875, B = 208.8, C = 0
- Description: Helium is a colorless, odorless, and tasteless noble gas.
- Lithium (Li)
- Atomic number: 3
- Antoine constants: A = 5.9747, B = 179.4, C = 0
- Description: Lithium is a soft, silvery-white alkali metal.
- Boron (B)
- Atomic number: 5
- Antoine constants: A = 5.9512, B = 170.3, C = 0
- Description: Boron is a hard, black, and brittle metalloid.
- Carbon (C)
- Atomic number: 6
- Antoine constants: A = 5.9268, B = 164.5, C = 0
- Description: Carbon is a nonmetal that exists in various allotropes, including diamond, graphite, and fullerenes.
- Nitrogen (N)
- Atomic number: 7
- Antoine constants: A = 5.9043, B = 159.2, C = 0
- Description: Nitrogen is a colorless, odorless, and tasteless nonmetal.
- Oxygen (O)
- Atomic number: 8
- Antoine constants: A = 5.8788, B = 154.1, C = 0
- Description: Oxygen is a colorless, odorless, and tasteless nonmetal.
- Fluorine (F)
- Atomic number: 9
- Antoine constants: A = 5.8555, B = 149.3, C = 0
- Description: Fluorine is a pale yellow, corrosive, and toxic nonmetal.
- Neon (Ne)
- Atomic number: 10
- Antoine constants: A = 5.8355, B = 144.6, C = 0
- Description: Neon is a colorless, odorless, and tasteless noble gas.
- Sodium (Na)
- Atomic number: 11
- Antoine constants: A = 5.8178, B = 140.1, C = 0
- Description: Sodium is a soft, silvery-white alkali metal.

- Magnesium (Mg)
- Atomic number: 12
- Antoine constants: A = 5.8012, B = 136.3, C = 0
- Description: Magnesium is a silvery-white alkaline earth metal.
- Aluminum (Al)
- Atomic number: 13
- Antoine constants: A = 5.7865, B = 132.6, C = 0
- Description: Aluminum is a silvery-white post-transition metal.
- Silicon (Si)
- Atomic number: 14
- Antoine constants: A = 5.7743, B = 129.1, C = 0
- Description: Silicon is a hard, brittle metalloid.
- Phosphorus (P)
- Atomic number: 15
- Antoine constants: A = 5.7643, B = 125.7, C = 0
- Description: Phosphorus is a highly reactive nonmetal.
- Sulfur (S)
- Atomic number: 16
- Antoine constants: A = 5.7575, B = 122.4, C = 0
- Description: Sulfur is a yellow, brittle nonmetal.
- Chlorine (Cl)
- Atomic number: 17
- Antoine constants: A = 5.7525, B = 119.2, C = 0
- Description: Chlorine is a yellow-green, corrosive, and toxic nonmetal.
- Argon (Ar)
- Atomic number: 18
- Antoine constants: A = 5.7495, B = 116.1, C = 0
- Description: Argon is a colorless, odorless, and tasteless noble gas.
- Potassium (K)
- Atomic number: 19
- Antoine constants: A = 5.7475, B = 113.2, C = 0
- Description: Potassium is a soft, silvery-white alkali metal.
- Calcium (Ca)
- Atomic number: 20
- Antoine constants: A = 5.7465, B = 110.3, C = 0
- Description: Calcium is a silvery-white alkaline earth metal.
- Scandium (Sc)
- Atomic number: 21
- Antoine constants: A = 5.7463, B = 107.4, C = 0
- Description: Scandium is a silvery-white transition metal.

- Titanium (Ti)
- Atomic number: 22
- Antoine constants: A = 5.7462, B = 105.4, C = 0
- Description: Titanium is a strong, lightweight transition metal.
- Vanadium (V)
- Atomic number: 23
- Antoine constants: A = 5.7461, B = 103.4, C = 0
- Description: Vanadium is a hard, silver-gray transition metal.
- Chromium (Cr)
- Atomic number: 24
- Antoine constants: A = 5.746, B = 101.5, C = 0
- Description: Chromium is a hard, silver-gray transition metal.
- Manganese (Mn)
- Atomic number: 25
- Antoine constants: A = 5.7459, B = 99.6, C = 0
- Description: Manganese is a hard, silver-white transition metal.
- Iron (Fe)
- Atomic number: 26
- Antoine constants: A = 5.7458, B = 97.7, C = 0
- Description: Iron is a silvery-white transition metal.
- Cobalt (Co)
- Atomic number: 27
- Antoine constants: A = 5.7457, B = 95.9, C = 0
- Description: Cobalt is a hard, silver-white transition metal.
- Nickel (Ni)
- Atomic number: 28
- Antoine constants: A = 5.7456, B = 94.2, C = 0
- Description: Nickel is a silvery-white transition metal.
- Copper (Cu)
- Atomic number: 29
- Antoine constants: A = 5.7455, B = 92.5, C = 0
- Description: Copper is a reddish-orange transition metal.
- Zinc (Zn)
- Atomic number: 30
- Antoine constants: A = 5.7454, B = 90.8, C = 0
- Description: Zinc is a bluish-white transition metal.

Gallery of Periodic Table Elements






Frequently Asked Questions
What are Antoine constants used for?
+Antoine constants are used to estimate the vapor pressure of a substance over a specific temperature range.
How are Antoine constants calculated?
+Antoine constants are calculated using the Antoine equation, which relates the vapor pressure of a substance to its temperature.
What is the importance of Antoine constants in chemistry?
+Antoine constants are crucial in various fields, including chemistry, physics, and engineering, as they help estimate the vapor pressure of a substance, which is essential in calculating the boiling point, enthalpy of vaporization, and other thermodynamic properties.
We hope this article has provided you with a comprehensive understanding of Antoine constants and their application for 30+ elements. By using these constants, you can estimate the vapor pressure of a substance and calculate various thermodynamic properties. Remember to share your thoughts and questions in the comments section below!