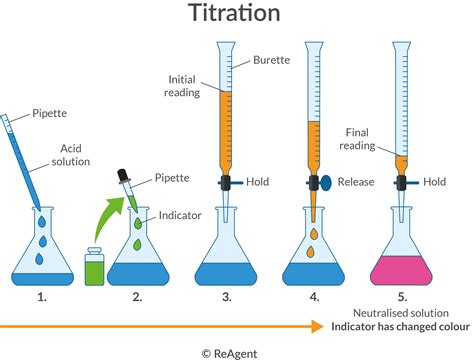
Acid-base titration is a widely used laboratory technique in various fields, including chemistry, biology, and environmental science. It involves the reaction of an acid with a base to form a salt and water, and is commonly used to determine the concentration of an unknown substance. But what are the real-life applications and uses of acid-base titration?
What is Acid-Base Titration?
Acid-base titration is a method of quantitative analysis that involves the reaction of an acid with a base. The acid is added to the base until the reaction is complete, at which point the concentration of the unknown substance can be calculated. The reaction is typically monitored using a pH indicator, such as phenolphthalein or methyl orange, which changes color when the reaction is complete.
Principle of Acid-Base Titration
The principle of acid-base titration is based on the neutralization reaction between an acid and a base. The acid donates a proton (H+ ion), while the base accepts a proton. The reaction is typically represented by the following equation:
HA + BOH → BA + H2O
Where HA is the acid, BOH is the base, BA is the salt, and H2O is water.

Real-Life Applications of Acid-Base Titration
Acid-base titration has numerous real-life applications in various fields, including:
Environmental Monitoring
Acid-base titration is widely used in environmental monitoring to determine the concentration of pollutants in water and air. For example, it can be used to measure the concentration of sulfuric acid in rainwater, which is a major contributor to acid rain.
Food and Beverage Industry
Acid-base titration is used in the food and beverage industry to determine the concentration of ingredients in food products. For example, it can be used to measure the concentration of citric acid in fruit juices.
Pharmaceutical Industry
Acid-base titration is used in the pharmaceutical industry to determine the concentration of active ingredients in medicines. For example, it can be used to measure the concentration of aspirin in tablets.
Biotechnology
Acid-base titration is used in biotechnology to determine the concentration of biomolecules in biological samples. For example, it can be used to measure the concentration of DNA in blood samples.
Uses of Acid-Base Titration
Acid-base titration has numerous uses in various fields, including:
Determination of Concentration
Acid-base titration is widely used to determine the concentration of unknown substances. By measuring the volume of acid required to neutralize a base, the concentration of the base can be calculated.
Quality Control
Acid-base titration is used in quality control to ensure that products meet the required standards. For example, it can be used to measure the concentration of ingredients in food products.
Research and Development
Acid-base titration is used in research and development to develop new products and processes. For example, it can be used to study the properties of new materials.
Forensic Science
Acid-base titration is used in forensic science to analyze evidence in crimes. For example, it can be used to analyze the concentration of substances in blood samples.

Limitations of Acid-Base Titration
While acid-base titration is a widely used technique, it has several limitations, including:
Interference from Other Substances
Acid-base titration can be affected by the presence of other substances that may react with the acid or base. For example, the presence of carbon dioxide in water can affect the pH of the solution.
Inaccurate Results
Acid-base titration can produce inaccurate results if the reaction is not complete or if the pH indicator is not accurate.
Time-Consuming
Acid-base titration can be a time-consuming technique, especially if the reaction is slow or if multiple titrations are required.

Conclusion
Acid-base titration is a widely used laboratory technique with numerous real-life applications in various fields, including environmental monitoring, food and beverage industry, pharmaceutical industry, and biotechnology. While it has several limitations, it remains a valuable tool for determining the concentration of unknown substances and ensuring quality control.
Gallery of Acid Base Titration






What is acid-base titration?
+Acid-base titration is a laboratory technique used to determine the concentration of an unknown substance by reacting it with a known substance.
What are the real-life applications of acid-base titration?
+Acid-base titration has numerous real-life applications in various fields, including environmental monitoring, food and beverage industry, pharmaceutical industry, and biotechnology.
What are the limitations of acid-base titration?
+Acid-base titration has several limitations, including interference from other substances, inaccurate results, and time-consuming process.